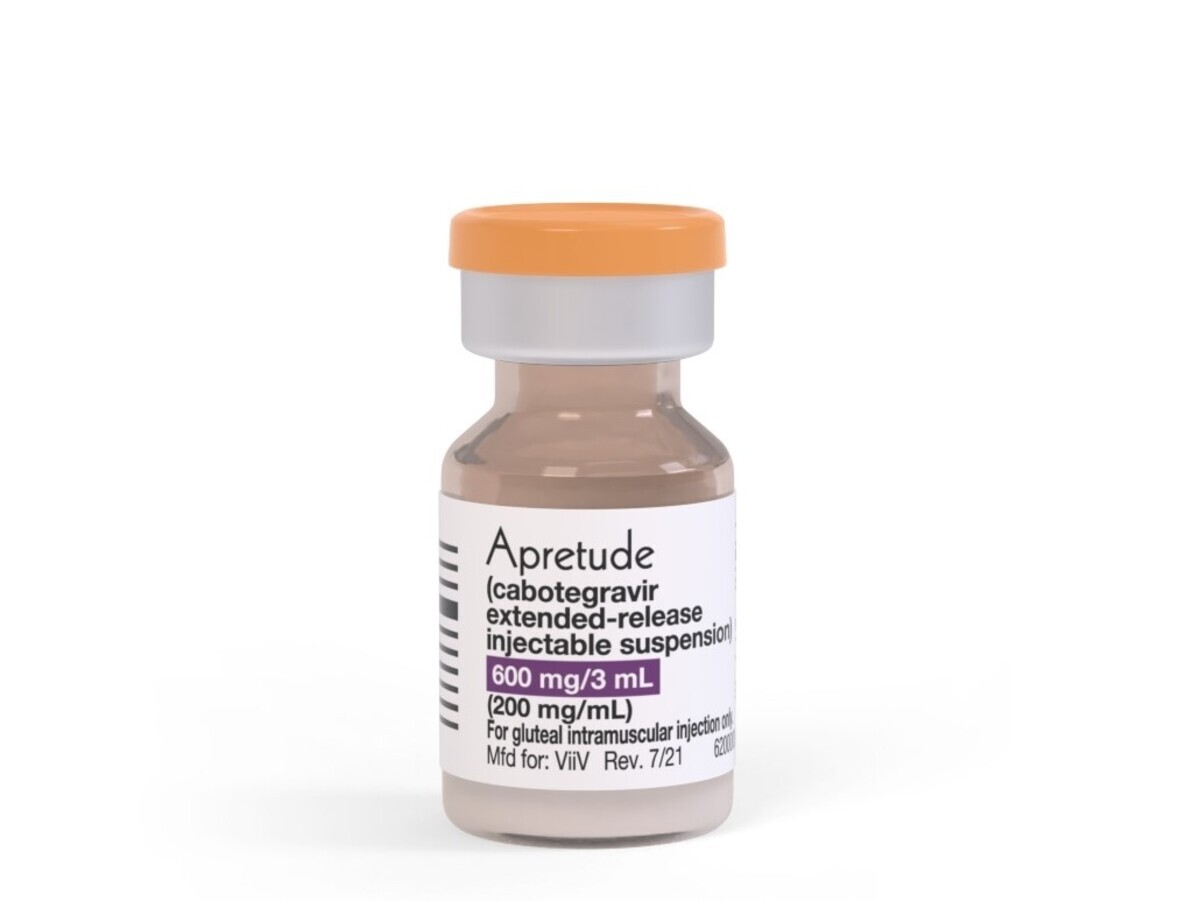
KUALA LUMPUR, Sept 15 – Malaysia’s Drug Control Authority (DCA) has endorsed the registration of Apretude, a cabotegravir-based medication, as a pre-exposure prophylaxis (PrEP) solution to protect high-risk groups from HIV infection.
The landmark approval covers two distinct formulations of Apretude – a tablet and a long-acting injectable (CAB-LA) – offering flexibility in HIV prevention strategies.
The decision follows a rigorous evaluation conducted under the ASEAN Joint Assessment programme, marking a first in DCA’s history, MOH pharmacy services director Norhaliza A. Halim said in a statement today.
Malaysia now joins an expanding list of countries where cabotegravir, marketed under the brand name Apretude, has received approval for use.
Apretude was first approved by the United States’ Food and Drug Administration (FDA) in December 2021, subsequently gaining approval in countries including Australia, Zimbabwe, South Africa, Malawi, Botswana, and Brazil.
Apretude’s key role as a PrEP solution is focused on reducing the risk of HIV-1 transmission, primarily for individuals at heightened infection risk. The medication is produced by Glaxo Operations (GSK) UK Ltd, based in the United Kingdom, and is registered under the oversight of GlaxoSmithKline Pharmaceutical Sdn Bhd in Malaysia.
The World Health Organization (WHO) released guidelines last year that urged countries to consider CAB-LA as an HIV prevention tool, describing it as “safe and highly effective”.
CAB-LA is an intramuscular injectable, long-acting form of PrEP, with the first two injections administered four weeks apart, followed thereafter by a jab every eight weeks. This is in contrast to the daily pills that characterise most PrEP regimes.
The MOH’s announcement came a year after infectious disease expert and former International AIDS Society (IAS) president Prof Dr Adeeba Kamarulzaman told CodeBlue that Malaysia will likely have access to generic versions of CAB-LA in 2027, along with other second-tier countries.
Norhaliza’s statement today did not indicate when and where CAB-LA would be made available in Malaysia. She simply said: “This product registration through the Asean Joint Assessment is the MOH’s initiative and commitment in continued efforts to increase and expedite Malaysians’ access to quality, safe and effective treatments.”
The MOH pharmacy services director added that the Asean Joint Assessment for Apretude was led by the Philippines, participated by Malaysia, Myanmar, Thailand, and Vietnam.
“The Asean Joint Assessment is a facilitated registration pathway where the evaluation of a product is conducted jointly by participating Asean regulatory bodies.
“Through this pathway, the company can make an application for product registration simultaneously to all these regulatory bodies. The joint product evaluation can then be used to consider registration approval in the respective countries,” Norhaliza said.
ViiV Healthcare, the HIV-focused unit of GSK, last year agreed to provide access to affordable generic versions of Apretude in 90 countries categorised as least developed, low-income, and lower middle-income, where over 70 per cent of all new HIV infections occurred in 2020.
The 90 countries include six Asean countries: Cambodia, Indonesia, Laos, the Philippines, Myanmar, and Vietnam. ViiV’s voluntary licensing deal with the Medicines Patent Pool (MPP) also grants selected manufacturers to produce generic versions of cabotegravir.
ViiV has further pledged that, until generic alternatives become available, it will provide the drug at a “non-profit price” for public programmes in low-income, least developed, and all sub-Saharan African countries. However, concerns remain that the pricing is too high for many nations.
Reports indicate that the cost of the injectable stands at US$3,700 (RM16,475) per dose. ViiV’s estimated not-for-profit price ranges from US$240 to US$270 for a year’s supply for a single patient, as reported by The Guardian. The Clinton Health Access Initiative’s calculations suggest that the actual ingredient costs may range from US$20 to US$40.