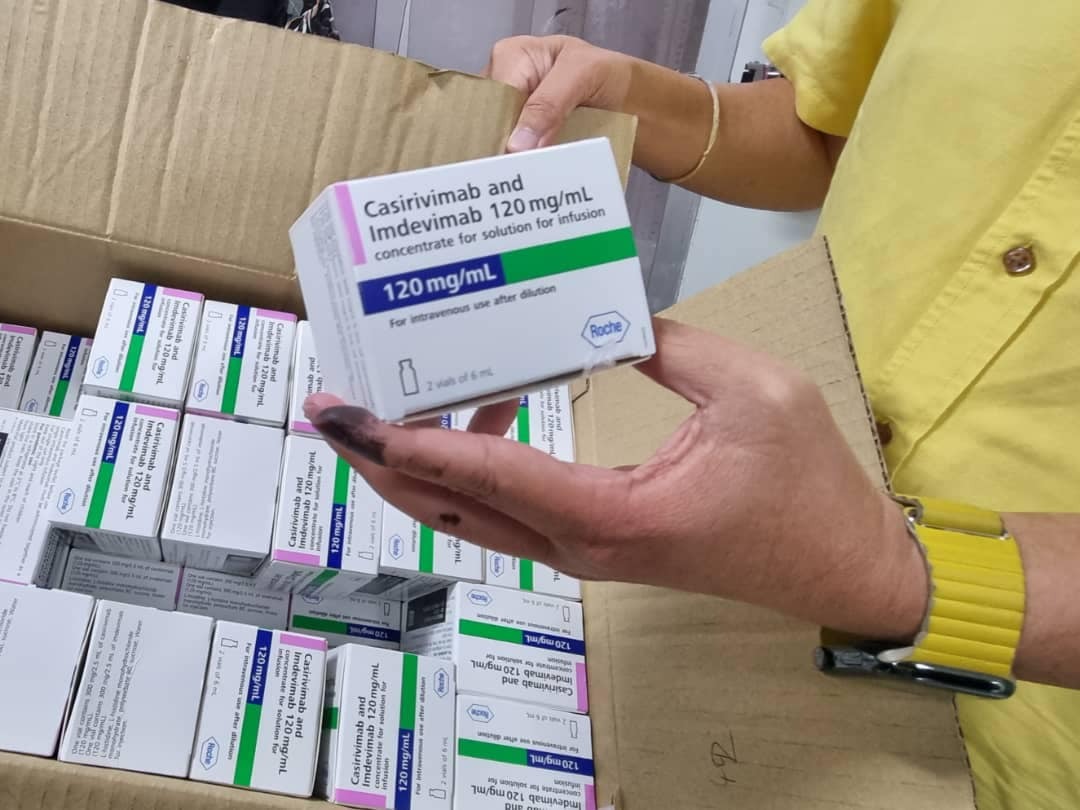
KUALA LUMPUR, Dec 22 – The Sarawak state government created history by purchasing the Ronapreve monoclonal antibody therapy for Covid-19, even before the federal administration bought supplies for government hospitals in other states.
Sarawak Local Government and Housing Minister Dr Sim Kui Hian said the state government was able to procure Ronapreve — which received conditional registration approval from the National Pharmaceutical Regulatory Agency (NPRA) on December 13 — due to engagements with Roche.
The monoclonal antibody cocktail was developed jointly by Renegeron, an American biotechnology company, and Swiss pharmaceutical company Roche.
“Truly historical as is first time life-saving drugs available in KKM public hospitals in Sarawak (not yet available in KKM public hospitals in rest of Malaysia) [and] purchased by state government, ahead of federal government (thanks KKM of federal government allow us to buy this new drugs for our people and to be administered in KKM hospitals in Sarawak),” Dr Sim posted on Facebook yesterday, referring to the Ministry of Health (MOH).
Dr Sim noted that the single-dose Ronapreve treatment costs a “few” thousand ringgit per dose. The Japanese government reportedly purchased Ronapreve at 310,000 yen (about US$2,730) per dose.
If the Sarawak state government paid the same price, it would amount to more than RM11,000 per dose.
Ronapreve — which is a cocktail of two monoclonal antibodies (casirivimab and imdevimab) designed to block infection of SARS-CoV-2 — will be used in Sarawak for Category Four Covid-19 patients, he said. Category Four is severe disease.
However, Health director-general Dr Noor Hisham said, when announcing NPRA’s conditional registration approval of Ronapreve, that the monoclonal antibody cocktail was authorised to treat Covid-19 in adults and adolescents (aged 12 years and older and weighing at least 40kg), who do not require supplemental oxygen and who are at increased risk of progressing to severe disease.
Ronapreve is also approved as a prophylactic to prevent Covid-19 in those aged 12 years and older, weighing at least 40kg, who have been exposed, or are at high risk of exposure to the coronavirus, and who either have a medical condition that may lessen their protection from vaccination or who have not received Covid-19 vaccines.
“The Ronapreve product is not meant to be used as a replacement for Covid-19 vaccination and public health measures, especially compliance with SOPs (standard operating procedures),” Dr Noor Hisham stressed.
Regulators in the United Kingdom and the European Union have approved Ronapreve for use, while the United States’ Food and Drug Administration (FDA) has issued emergency-use authorisation for use of the monoclonal antibody treatment (known as REGEN-COV in America) to treat mild-to-moderate Covid-19.
The FDA has also authorised the monoclonal antibody therapy to prevent Covid-19 after exposure to the SARS-CoV-2 virus in those aged 12 years and older who are at high risk of progressing to severe Covid-19.
Regeneron said in a November 30 press release that early testing of Ronapreve indicated possible reduced efficacy against the new Omicron variant.