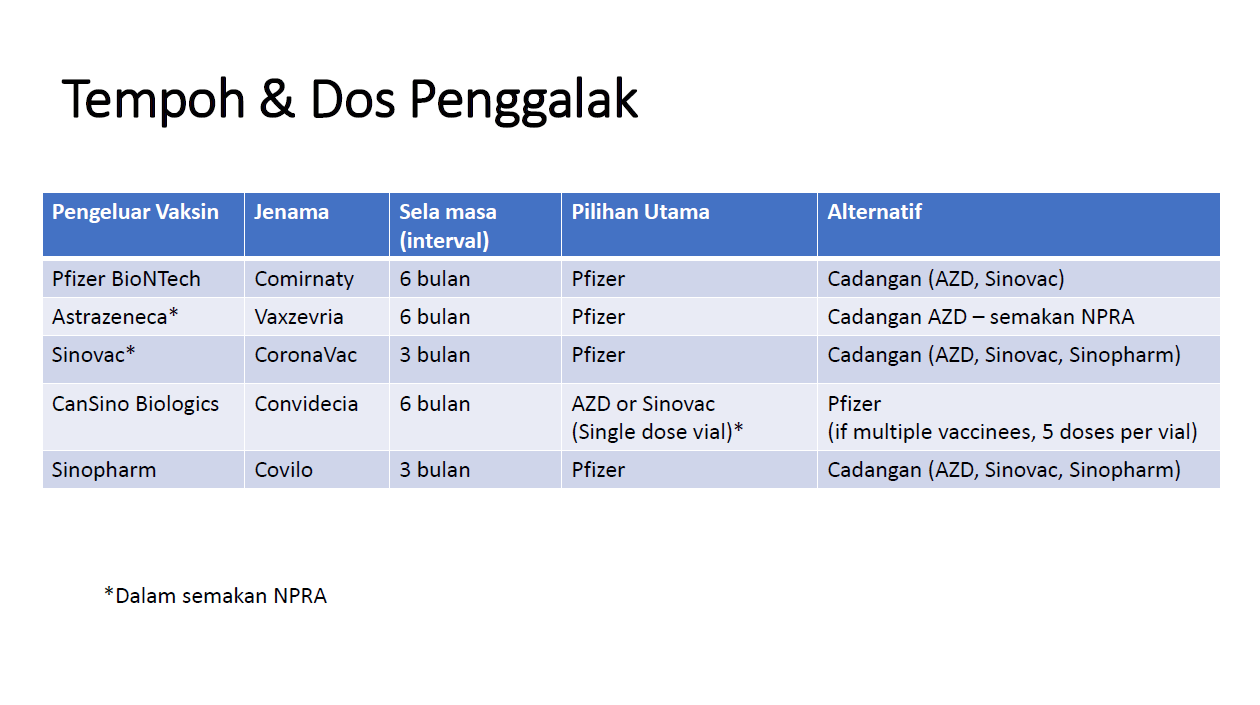
KUALA LUMPUR, Nov 12 — The Ministry of Health (MOH) recommends a third Pfizer Covid-19 shot for recipients double vaccinated with the AstraZeneca and Sinopharm vaccines, based on studies that show higher efficacy in individuals that received a third heterologous Pfizer dose.
Individuals double vaccinated with AstraZeneca are recommended to get a third Pfizer jab within six months after the second dose, while those who are fully vaccinated with Sinopharm should get a third shot with Pfizer within three months after their second Sinopharm jab.
The MOH also recommends a third Pfizer shot for Sinovac recipients three months after the second Sinovac dose.
For individuals vaccinated with a single CanSino dose, the MOH is recommending a second shot with either a single AstraZeneca or Sinovac dose, pending review and approval from the National Pharmaceutical Regulatory Agency (NPRA).
The MOH also lists AstraZeneca, Sinovac, and Sinopharm Covid-19 vaccines as alternative boosters for both homologous and heterologous vaccination pending NPRA review.
The NPRA has only authorised the Pfizer mRNA vaccine as a third dose for Pfizer recipients or as part of heterologous vaccination for individuals who received two Sinovac doses.
Health director-general Dr Noor Hisham Abdullah said the NPRA is still evaluating the use for homologous or non-Pfizer boosters for AstraZeneca and Sinovac recipients.
“Both AstraZeneca and Sinovac have submitted their dossier and we are in the process of reviewing it. The next meeting for the Drug Control Authority (DCA) will be on November 17. We need time to review the data, so, this will be under the NPRA,” Dr Noor Hisham told a media briefing today.
Dr Noor Hisham said while it is possible for Sinovac recipients to receive a third Sinovac shot, pending review by the NPRA, he noted that recently published studies in countries that widely use Sinovac’s Covid-19 vaccine pointed to higher vaccine efficacy in heterologous vaccination.
“Yes, it’s possible to receive (a Sinovac booster shot for Sinovac recipients) but at the moment the NPRA has not approved the Sinovac booster yet. They’ve just submitted the booster [dossier] to NPRA so we are reviewing it.
“So, today we know the data comes from Thailand, Chile, and Turkey. So, we are doing a one year study, in terms of collection of the data [for] Sinovac-Sinovac-Pfizer.
“We hope within one year, we will get the data and we can actually contribute [to the global data]. Most of the studies done in Chile, Turkey, and Thailand show that efficacy has increased better than homologous Sinovac-Sinovac-Sinovac.
“We will make a decision under NPRA, the dossier has been submitted so we hope we can get approval as soon as possible,” Dr Noor Hisham said.
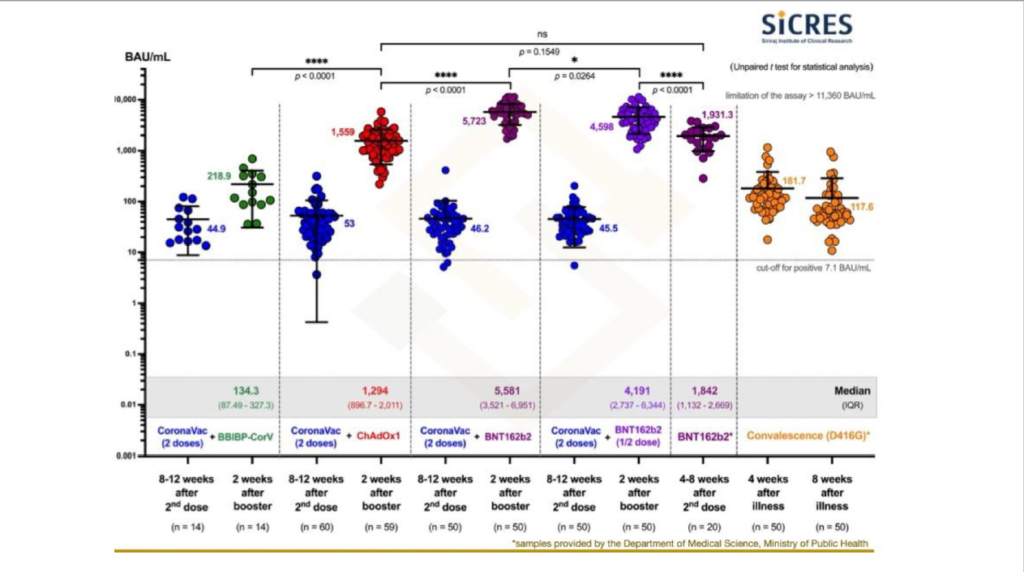
An updated version of Chile’s study showed higher efficacy with Pfizer or AstraZeneca as boosters for Sinovac recipients compared to a third Sinovac jab in preventing SARS-CoV-2 infection, symptomatic disease, hospital admissions, and intensive care unit (ICU) admissions.
The “Covid-19 vaccine effectiveness assessment in Chile” study released by the Chilean government at a World Health Organization (WHO) consultation on October 25, showed that efficacy in preventing SARS-CoV-2 infection — among individuals double vaccinated with Sinovac — rose to about 93 per cent for a third dose with Pfizer, 91 per cent for AstraZeneca, and 71 per cent for Sinovac.
To compare, the 71 per cent efficacy rate for a third Sinovac jab, 14 days after the third shot, was lower than 81 per cent efficacy with two Pfizer doses, 14 days after the second dose.
The Thai study, conducted on volunteers who had received two Sinovac doses, also found that a full third dose of Pfizer induced the highest boosting effect, followed by a half dose of Pfizer. Sinopharm’s Covid-19 vaccine had the lowest boosting effect, the study noted.
It further pointed out that a third AstraZeneca Covid-19 vaccine produced a boosting effect that is comparable to the level at a four to eight-week post-primary series of two doses of the Pfizer vaccine.
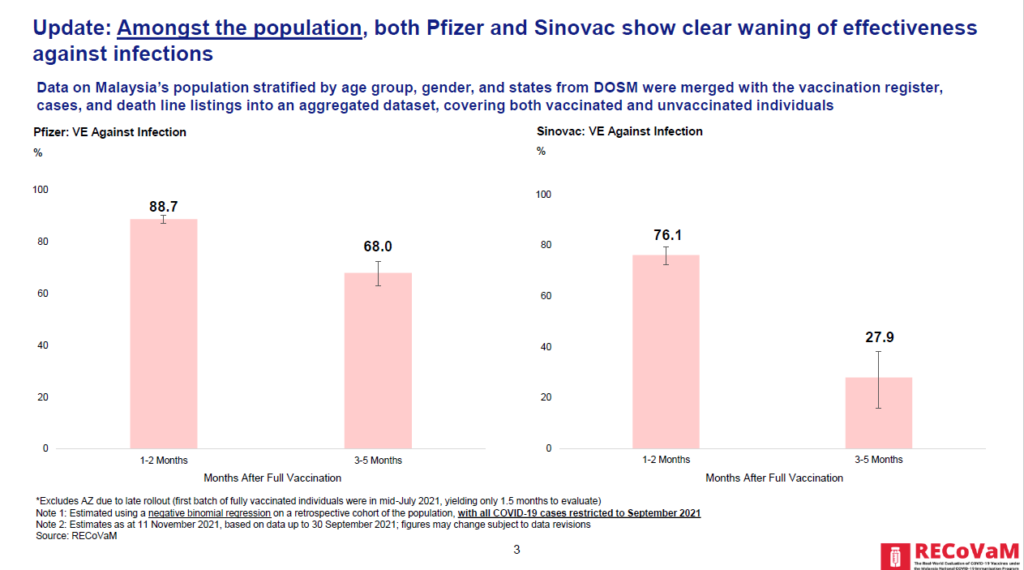
Institute of Clinical Research (ICR) director Dr Kalaiarasu Peariasamy, who was also present during the media briefing, said that data from the Real-World Evaluation of Covid-19 Vaccines Under the Malaysia National Covid-19 Immunisation Program (RECoVaM) study found that both Pfizer and Sinovac showed clear waning of effectiveness against Covid-19 infections over time.
However, RECoVaM data showed that Sinovac had lower vaccine efficacy against infection compared to Pfizer, with Sinovac recording an efficacy rate against infection of 76.1 per cent within the first two months after full vaccination and 27.9 per cent after 3 to 5 months.
Pfizer’s Covid-19 vaccine efficacy rate against infection stood at 88.7 per cent in the first two months after full vaccination before it declined to 68 per cent after three to five months.