Interest in Covid-19 antibody testing arose from the public concern of their immune status following natural infection of the virus or post-vaccination.
This was related to reports of several incidences where empty syringes (without vaccine) might have been used during mass vaccination program at several vaccination centres.
Another puzzling question is whether the expected antibody levels are protective. Does absence of antibody against SARS-CoV-2 or a waning level imply risk to infection?
A loss of antibody is a warning sign that vaccine may wear off, but without any indication when the loss actually begins. A waning antibody level with time is expected with immunisation as the immune response has been documented to successfully reduce the viral load and hence do not pose as a threat.
However, the protective immune system creates a backup strategy in the form of memory cells generated from the known immune cells of T and B types. Should subsequent similar threats appear from the original attacking virus, antibody formation will be accelerated and heightened.
Thus, to address these and other related questions on antibody testing, it is imperative that we understand the basics of the immune response to this SARS-CoV-2 virus.
How Is The Antibody Produced In The Body?
An entry of a germ, SARS-CoV-2 virus as an example, into the human body will elicit an immune response. The immune system will mount a defensive immune response as the virus is not recognised as self, and treat the virus as a threat and harmful by producing antibodies to act against it.
The triggered immune response is a very specific biological event leading to the production of antibodies against various viral identity markers such as the spike proteins (commonly known as the viral antigen) found on the surface of the virus particle.
Besides the release of antibodies, our body also produces cells specifically to eliminate the virus known as the cytotoxic T-cells (CD8+ as the identifiable marker of the cell). T-cell response to SARS-CoV-2 spike protein has been shown to be present in almost all Covid-19 patients.
Likewise, B-cells showing a similar response, evidence of working relationship among these immune cells to get rid of the virus. It is very unlikely that the presence of SARS-CoV-2 antibody in an unexposed person is the result of cross-reactivity to previous common cold from other types of coronavirus infections.
Studies of T-cell response in infected individual revealed SARS-CoV-2-specific CD8+ T-cells as well as CD4+ T-cells (another type of T-cell that plays an important role in assisting the production of Covid-19 antibodies) were maintained over 10 months post infection.
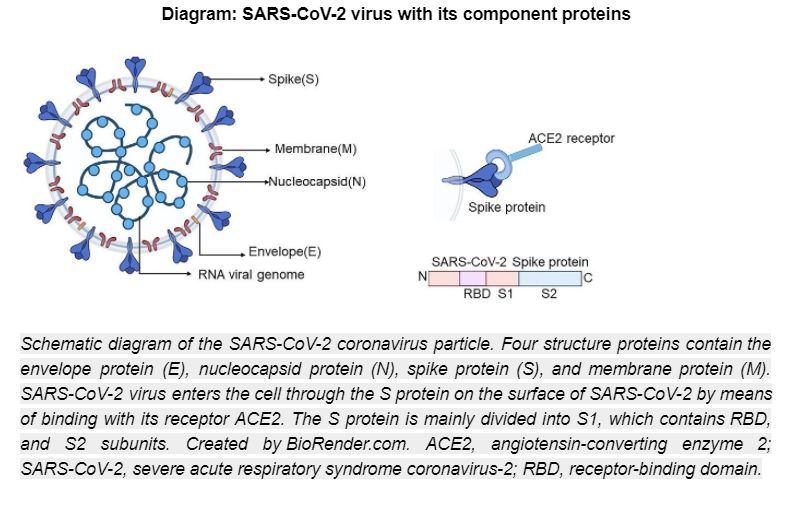
The Covid-19 virus being foreign to our body is an example of an antigen that will activate the immune response. The SARS-CoV-2 virus contains four major structural proteins, namely spike (S), membrane (M) and envelope (E) proteins, which are embedded on the viral surface envelope, and nucleocapsid (N) protein, which is found within the inner core of the virus.
The immune response will result in antibody production and acts by neutralisation against the receptor binding domain (RBD), one of the sub-units of the spike protein structure.
The RBD specific antibodies contribute to 90 per cent of the total neutralisation activity in human blood following Covid-19 infection, Antibodies are formed and detectable in post-infected and post-vaccinated individuals.
Following vaccination to a specific Covid-19 vaccine, the serum is likely to show antibodies intended to be produced according to the type of vaccine. The approved vaccines were manufactured differently but with the intention of resulting in the formation of protective or neutralising antibodies:
- mRNA vaccines (Pfizer-BioNTech and Moderna) are aimed at producing the viral spike proteins via utilization of our own body cells machinery. The presence of the viral spike proteins on our own cells will subsequently stimulate antibody production as the proteins are recognised to be foreign to our body defense system.
- These protective antibodies will provide instant defense in any event our body encounters an attack from the COVID-19 virus. In mRNA vaccine recipients, antibodies towards the M, E, and N proteins are unlikely to be formed or detected.
- Viral vector vaccines (AstraZeneca, J&J, CanSino Bio). In this type of vaccine, a piece of genetic material from the Covid-19 virus is placed in a modified version of a different virus (viral vector). When the viral vector gets into our cells, it gives instructions to the cells to make copies of the S protein. Once our cells display the S proteins on their surfaces, the immune system responds by creating antibodies against the S protein and not the M, E or N proteins. So, this specific antibody will fight the Covid-19 virus should we become infected with the virus later.
- Sinovac Biotech vaccine is an inactivated vaccine in which the Covid-19 virus is killed or modified that it will not be able to replicate or multiply nor cause disease when injected into our body. This type of vaccine contains most of the SARS-CoV-2 protein components that will trigger the immune system to generate a response. Therefore, other than production of antibodies against the specific S protein, the vaccine recipients may also produce antibodies against the N and E proteins.
With this information, antibody test kit determining spike protein specific antibodies can be used to measure antibody level production in individual vaccinated with Pfizer, AstraZeneca or Sinovac vaccines. With the exception of Sinovac vaccine recipients, the presence of SARS-CoV-2 specific antibodies against the M, N or E may suggest a prior infection of the individuals with Covid-19.
FAQ On Antibody Levels In Covid-19
Are the antibodies against SARS-CoV-2 protective?
A person who has detectable antibodies to SARS-CoV-2 is called SARS-CoV-2 seropositive. Seronegative means that a person does not have detectable SARS-CoV-2 antibodies. Those SARS-CoV-2 seropositive individuals may have decreased risk of future infection. A specific-antibody towards the RBD of spike proteins has been shown to neutralise the virus in the laboratory, and it is believed to provide protection.
Does natural infection induce immunity that is more protective than that induced by vaccines?
As far as COVID-19 is concerned natural immunity confers longer lasting and stronger protection against infection, symptomatic disease and hospitalisation caused by the Delta variant of SARS-CoV-2, compared to vaccine-induced immunity. However, this does not mean that vaccination is not protective and that we should risk ourselves to be infected. As we are all aware, natural infection can also cause severe disease and death.
How long does antibody to SARS-CoV-2 last?
Antibodies against SARS-CoV-2 may not be detectable 0-14 days after vaccination or infection. Antibody levels will start to peak at around 2-4 weeks post vaccination, which will gradually reduce with time. It is observed that the total RBD-specific antibodies gradually decrease between 6-31 weeks after vaccination.
What is the durability of protection?
Neutralising Covid-19 antibodies persist longer following infection, with data showing that natural infection produce immunity lasting more than six months. In addition, neutralising antibodies to the spike (S) protein persisted at high levels until 13 months post-infection, when it declines. Severe disease is associated with higher positivity rates over time i.e., a more robust antibody response. However, scientists are still investigating what is the range of antibody levels that will confer protection. This would probably vary from one individual to another due to other factors like other immune parameters and comorbidities.
How do various vaccines affect effectiveness?
Various vaccines have reported efficacies that ranges from 50% to 95%. However, it should be noted that all the vaccines have been tested on different populations and have not been compared head-to-head within the same study. For example, the Sinovac vaccine recorded an efficacy of a little above 50% among health care workers in Brazil while the same vaccine recorded more than 80% efficacy among the healthy volunteers in Turkey. Since healthcare workers are highly exposed to infected individuals, especially during the peak wave of infection in Brazil, they were more likely to be infected. However, all vaccinated individuals in that study did not experience severe disease or require hospitalisation. Currently, all the vaccines that have been rolled out in Malaysia and elsewhere are safe and effective.
What about vaccination for individuals with previous Covid-19 infection?
As mentioned above, individuals who have previously been infected already have good immunity against COVID-19. Hence, they may not need the full 2-dose vaccination. Importantly, vaccination for previously infected individuals is safe even if 2 doses of the vaccine is given.
Presence of SARS-CoV-2 antibodies in our body occurs as a result of introduction of such virus either from natural infection or through immunisation.
Antibody to SARS-CoV-2, especially towards the spike protein, is protective against future infection. It may not be detectable earlier than two weeks of infection or vaccination with peaks within two to four weeks. The level had been shown to have declined by six weeks post vaccination, but still detectable within 13 months post-infection.
More than 90 per cent of individuals with prior SARS-CoV-2 infections have higher antibody levels specific to the spike protein at all times compared to non-infected individuals. It offers a protection up to six months and perhaps longer.
Hence, a single dose of mRNA vaccine is all that is needed in patients with past infection.
References
- Heinz FX, Stiasny K. Distinguishing features of current COVID-19 vaccine: knowns and unknowns of antigen presentation and modes of action. NPJ Vaccines 2021; 6:104. doi: 10.1038/s41541-021-00369-6
- Harvey RA, Rassen JA, Kabelac CA, Turenne W, Leonard S, Klesh R, et al. Association of SARS-CoV-2 seropostive antibody test with risk of future infection. JAMA Intern Med 2021; 181: 672. doi: 10.1001/jamainternmed.2021.0366
- Sivan Gazit, Roei Shlezinger, Galit Perez, Roni Lotan, Asaf Peretz, Amir Ben-Tov, et al. Comparing SARS-CoV-2 natural immunity to vaccine-induced immunity: reinfections versus breakthrough infections. medRxiv preprint. doi: https://doi.org/10.1101/2021.08.24.21262415
- Anand SP, Prevost J, Nayrac M, Beudoin-Bussieres G, Benlarbi M, Gasser R, et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individual up to 8 months post-symptom onset. Cell Rep Med 2021; 2: 100290
- Wisnivesky JP, Stone K, Bagiella E, Doernberg M, Mendu DR, Lin JJ, Kale M. Long-term persistence of neutralising antibodies to SARS-Cov-2 following infection. J Gen Intern Med 2021. https://doi.org/10.1007/s11606-021-07057-0
- Favresse J, Bayart J-L, Mullier F, Elsen M, Eucher C, van Eeckhoudt S, et al. Antibody titres decline 3-month post-vaccinatiom with BNT162b2. Emerg Microbes Infect 2021; 10: 1495. doi: 10.1080/22221751.2021.1953403
- Ebinger J, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nature Medicine 2021; 27: 981. https://doi.org/10.1038/s41591-021-01325-6
This feature is courtesy of Translational Immunology Group for Education, Research and Society (TIGERS), and is jointly written by Dr Amir Hamzah Abdul Latiff, Dr Lokman Mohd Noh, Prof Dr Rahim Mohd Noah, Dr Adli Ali, Dr Norazmi Mohd Nor, Dr Intan Hakimah Ismail and Prof Dr Zamberi Sekawi.
- This is the personal opinion of the writer or publication and does not necessarily represent the views of CodeBlue.





