KUALA LUMPUR, March 23 — The Drug Control Authority (DCA) has cancelled the registrations of and recalled all cough and cold medications containing pholcodine, a cough suppressant, from the Malaysian market.
Malaysia’s decision came following similar withdrawals in the United Kingdom, Australia, and a recommendation for withdrawal by the European Medicines Agency (EMA) for the European Union (EU) market.
Malaysia’s DCA cited findings that people who took medications containing pholcodine in the past 12 months were at risk of developing anaphylaxis — a severe life-threatening allergic reaction that could occur within seconds or minutes after exposure to the irritant — with the administration of muscle relaxants or neuromuscular blocking agents (NMBAs) used in general anaesthesia.
“In other words, those who took pholcodine (usually cough medicine) in the past 12 months face a higher risk of anaphylaxis if they are given muscle relaxants or neuromuscular blocking agents (NMBAs) when undergoing general anaesthesia, for example during surgery,” Health director-general Dr Noor Hisham Abdullah said in a statement.
Pholcodine is an opioid medicine previously approved for use in adults and children to treat non-productive (dry) cough. In Malaysia, pholcodine is classified as a Class C controlled drug, meaning it can be sold without a prescription.
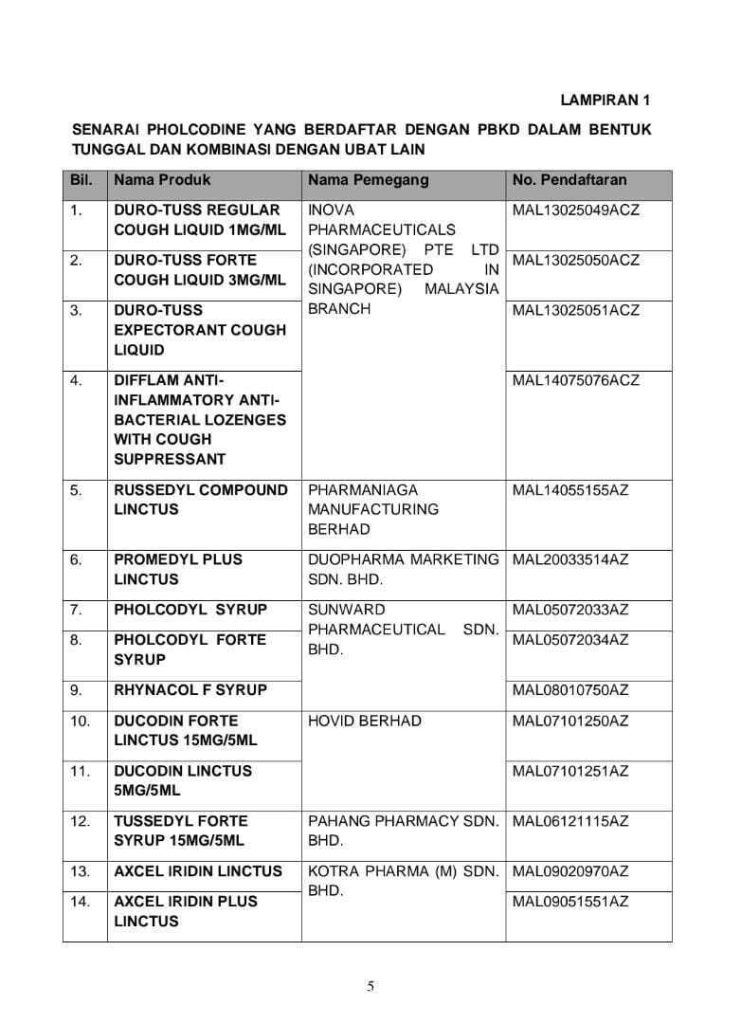
Dr Noor Hisham stated that 14 products containing pholcodine are registered with the DCA. The registered medication consists of medication only containing pholcodine or medication where pholcodine is mixed with other types of drugs.
“So far, the National Centre for Adverse Drug Reactions Monitoring, National Pharmaceutical Regulatory Agency (NPRA) Section, has received a total of 12 reports with 17 adverse effects following the use of pholcodine. However, amongst the reports received, no reports of anaphylaxis were received.”
The Health DG, however, cited the World Health Organization (WHO) that has reported 852 cases of adverse effects linked to the use of pholcodine. Amongst the reported cases, 42 reports of anaphylactic reaction and 20 reports of anaphylactic shock were recorded. From those cases, there were nine cases that involved the joint use of the NMBA suxamethonium, a short-acting muscle relaxant.
“With that, based on a thorough risk assessment, it is found that there are no steps to lower the risk that can be taken to minimise the risk for the user. Subsequently, it is found that the risk of taking medication containing pholcodine is higher than the benefits.
“Accordingly, the DCA has agreed to revoke the registration of all products containing pholcodine and carry out the recall procedure,” Dr Noor Hisham said.
“For [your] information, there are alternative medicines that can be used for non-productive/ dry, irritating cough, such as products that contain dextromethorphan in the single form and in combination with other drugs that are registered with the DCA.”
All product registration holders (PRH) are responsible for informing community-based pharmacies and clinics that have been supplied with the recalled products containing pholcodine to halt sales and to quarantine the product before returning it to the supplier.
Medical staff are also reminded to immediately stop prescribing, dispensing, selling, or distributing the product, and to give patients with dry cough alternative medication.
All stock balances of medication containing pholcodine must be quarantined and returned to the supplier.
The NPRA has also issued a Safety Alert titled Pholcodine: Risk of Anaphylaxis to Neuromuscular Blocking Agents (NMBAs) dated 9 March 2023 (updated on 23 March 2023) on NPRA’s official website to ensure the safety issues with the use of pholcodine can be given due attention.
As for the general public, Dr Noor Hisham reminded people who are taking cough medication to check the label or the insert in the product packaging of the cough medication for pholcodine.
If the active ingredient pholcodine is found, people should stop taking the medication and go to a health personnel and seek alternative treatment.
For members of the public who need surgery and require full anaesthesia, they are required to inform health personnel if they took medication containing pholcodine, especially if they took it in the past 12 months.
“MOH encourages the public to report any suspected adverse effects to the NPRA. Reporting of adverse effects can be done online by filling out the report form for users which is the Consumer Side Effect Reporting Form (ConSERF) that can be accessed at the NPRA website.”








