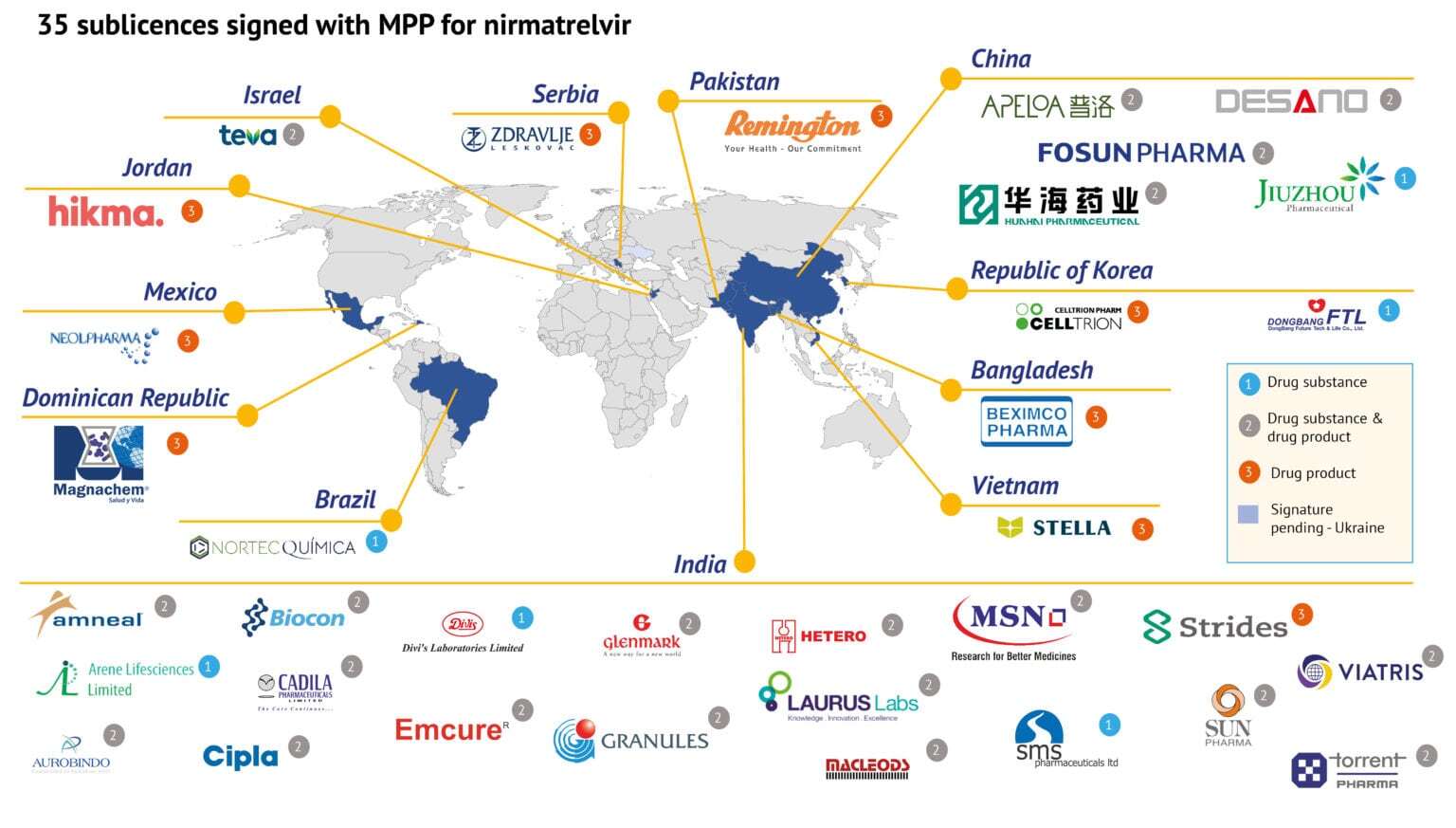
KUALA LUMPUR, March 22 – Malaysia was not selected to manufacture low-cost generic versions of Pfizer’s Covid-19 oral antiviral treatment, with agreements signed instead with manufacturers from a dozen other countries.
The United Nations-backed Medicines Patent Pool (MPP) announced last Thursday that it has signed agreements with 35 companies from 12 countries — Bangladesh, Brazil, China, Dominican Republic, Jordan, India, Israel, Mexico, Pakistan, Serbia, South Korea, and Vietnam — to produce the generic version of nirmatrelvir, in combination with ritonavir.
Pfizer’s Covid-19 medication, sold under brand name Paxlovid, comprises two drugs: nirmatrelvir, which is an antiviral developed by the American pharmaceutical company that inhibits the SARS-CoV-2 protein from replicating, and ritonavir, generally used to treat HIV, but is also used to boost levels of antiviral medicines.
MPP — which is a public health organisation that works to increase access to life-saving medicines for low- and middle-income countries — also offered a licence to a 36th company in Ukraine, but it has not been able to sign due to the ongoing war.
The non-exclusive sublicences to the 35 companies are the result of a voluntary licensing agreement signed by MPP and American pharmaceutical company Pfizer last November to help supply the medicine to 95 low- and middle-income countries that comprise about 53 per cent of the world’s population.
“The companies that were offered the sublicence demonstrated their ability to meet MPP’s requirements related to production capacity, regulatory compliance, as well as international standards for quality-assured medicines,” MPP said in a statement.
Under the sublicence agreements, six companies will produce the raw ingredients for nirmatrelvir, nine will co-package it with ritonavir for the finished product, and the remaining countries will do both.
“Nirmatrelvir is a new product and requires substantial manufacturing capabilities to produce, and we have been very impressed with the quality of manufacturing demonstrated by these companies,” said MPP executive director Charles Gore.
According to MPP, Pfizer will not receive royalties from sales of nirmatrelvir from MPP sublicencees while Covid-19 remains designated by the World Health Organization (WHO) as a Public Health Emergency of International Concern (PHEIC).
After the pandemic period, lower-middle income and upper-middle income countries will be subject to 5 per cent and 10 per cent royalties for sales to the public sector and the private sector respectively; sales to low-income nations will remain royalty free.
Clinical trials showed that Paxlovid reduced the risk of hospitalisation or death by 89 per cent in non-hospitalised high-risk adults with Covid-19 who were treated within three to five days of symptom onset.
Under the United States’ Food and Drug Administration’s (FDA) emergency-use approval for Paxlovid — which is administered as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) taken together twice daily for five days — the medication is authorised to treat mild to moderate Covid-19 disease in those aged 12 years and older who weigh at least 40kg, and who are at high risk of progressing to severe Covid-19.
“We have established a comprehensive strategy in partnership with worldwide governments, international global health leaders and global manufacturers to help ensure access to our oral Covid-19 treatment for patients in need around the world,” Pfizer chairman and CEO Albert Bourla said in MPP’s statement.
“The MPP sublicensees and the additional capacity for Covid-19 treatment they will supply will play a critical role to help ensure that people everywhere, particularly those living in the poorest parts of the world, have equitable access to an oral treatment option against Covid-19.”
Pfizer previously said in a November 2021 statement that “qualified” generic medicine manufacturers worldwide were granted sub-licences to supply Paxlovid under the head licence agreement between Pfizer and MPP.
Malaysia’s National Pharmaceutical Regulatory Agency (NPRA) has issued conditional approval for use of Paxlovid to treat Covid-19 in adults aged 18 and above who do not need oxygen therapy and are at high risk of developing severe symptoms.
Health Minister Khairy Jamaluddin previously said that Malaysia is expected to receive an initial shipment of 110,000 treatment courses of Paxlovid this month. NPR reported that the United States government is paying about US$530 (RM2,228) for each five-day course of Pfizer’s Covid-19 pill. Prices for the Malaysian government are unknown.
Why Malaysia Was Not Selected For Generic Manufacturing

Galen Centre for Health and Social Policy chief executive Azrul Mohd Khalib surmised three reasons as to why MPP did not sign an agreement with a Malaysian pharmaceutical company to produce generic versions of Pfizer’s Covid-19 pill.
He said there may already be sufficient manufacturers and Malaysia might still be considered in future if the need exceeds supply.
The second reason is that many of the countries selected for generic manufacturing of Pfizer’s Covid-19 treatment have a competitive advantage over Malaysia, due to their mature and well-developed pharmaceutical industries that can produce drugs at very high volume and with consistent quality.
“Recently, Pharmaniaga faced significant challenges in implementing the fill-finish process of the Sinovac vaccine, which may demonstrate that there remains capacity gaps at the domestic gaps which need to be overcome,” Azrul told CodeBlue.
A report by Parliament’s Public Accounts Committee (PAC) on Malaysia’s Covid-19 vaccine procurement revealed that the government was forced to pay a premium for imported Sinovac vaccine doses from China, due to delayed deliveries of the fill-and-finish product of the inactivated vaccine produced by local pharmaceutical company Pharmaniaga Berhad.
“Thirdly, Malaysia’s position and continuous action regarding the use of a government-use licence to acquire generic versions of sofosbuvir to treat Hepatitis C may have disadvantaged it from consideration by the US-based pharmaceutical company,” Azrul added.
In 2020, the Pharmaceutical Research and Manufacturers of America (PhRMA), a trade group representing companies in the US pharmaceutical industry, singled out Malaysia for purportedly undermining intellectual property protection, citing Malaysia’s government-use licence for sofosbuvir, a breakthrough innovation drug by American biopharmaceutical company Gilead Sciences.
Malaysia used the government-use licence to obtain generics of the Hepatitis C cure without the drug maker’s consent.
Azrul said the Malaysian pharmaceutical industry’s manufacturing capability and capacity are of high quality and of regulatory compliance, and meet good manufacturing practices as per international standards.
“However, the other countries have a significant advantage compared to Malaysia due to the size of their manufacturing capacity and years of experience producing such medical products at scale.”
He expects Malaysia to acquire the original Paxlovid drug directly from Pfizer at first, before obtaining the cheaper generic version once acquisition frameworks are finalised and open for procurement.