KUALA LUMPUR, Dec 28 – The Covid-19 Immunisation Task Force (CITF) has shortened the interval period for coronavirus vaccine boosters from six to three months for individuals initially vaccinated with AstraZeneca or Pfizer.
Health Minister Khairy Jamaluddin cited clinical and real-world data from the local Recovam study by the Institute for Clinical Research that found waning vaccine efficacy against Covid-19 infection.
He also cited latest data that shows the Omicron variant of concern is more transmissible than previous variants and has higher immune escape from vaccines or previous infection.
Regulatory bodies in the United Kingdom, Canada, and Australia, besides medical experts, also recommend Covid-19 booster shots as early as three months from primary vaccination.
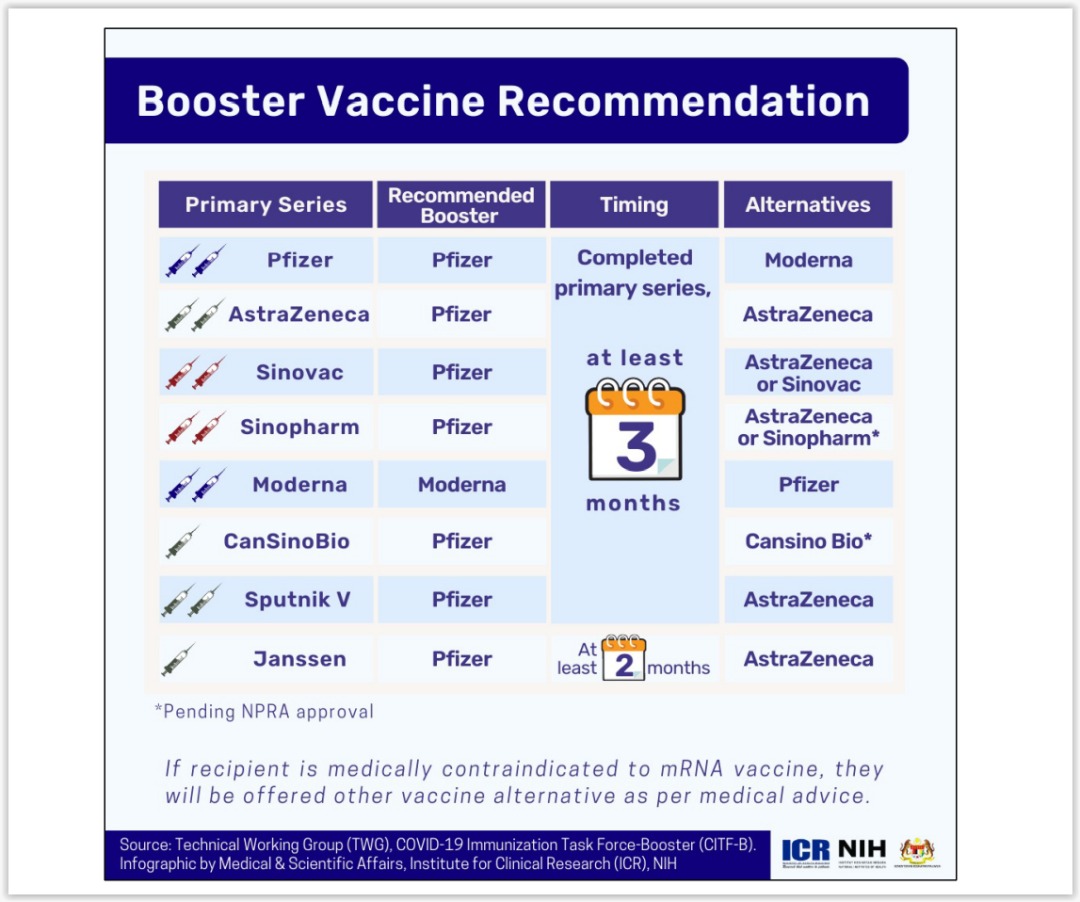
As such, CITF’s Technical Working Group now recommends that fully vaccinated adults aged 18 and older receive their boosters at least 90 days after their primary vaccine series. This applies to people who were initially inoculated with Pfizer, AstraZeneca, Sinovac, Sinopharm, Moderna, CanSino, or Sputnik V.
Those who received the single-shot Johnson & Johnson vaccine can get a booster after at least two months.
Booster vaccination appointment dates will be given according to age, where those aged 50 years and above will be offered their additional vaccine dose first, followed by over-40s, and then those aged 18 years and above.
Individuals with comorbidities, as well as residents and workers at long-term care facilities, will also be prioritised in the booster campaign.
“The Ministry of Health (MOH) expects the majority of adults who are eligible for boosters to receive their boosters in January and February,” Khairy told a press conference today, adding that more vaccination centres (PPVs) would be opened up – whether in private clinics or halls – to increase capacity.
Khairy stressed that MOH’s first choice for boosters is Pfizer-BioNTech’s mRNA vaccine, with AstraZeneca’s viral vector shot as an alternative, for all adults regardless of the type of vaccine taken for their primary series.
“Sinovac will only be given to contraindicated people because data shows Pfizer and AstraZeneca vaccine efficacy as a booster is very very high, compared to Sinovac.”
Only individuals who are allergic to either Pfizer and AstraZeneca can receive a Sinovac booster for free under the government’s National Covid-19 Immunisation Programme (PICK). Those without such contraindications can pay for a Sinovac booster in the private market.
Khairy cited encouraging data from Sarawak that leads the booster vaccination campaign, with 53.5 per cent of its adult population having received third doses to date, or 1,093,421 people.
Confirmed Covid-19 cases in Sarawak have been declining over the past two months, from 776 in the 48th epidemiological week to 208 in the 52nd week.
Covid-19 patients in the intensive care unit (ICU) in Sarawak declined significantly from 99 cases on November 5 to 11 on December 26. Deaths caused by Covid-19 also dropped in the East Malaysian state from 497 fatalities in October to 24 this month up to December 28.
“The decline in reported Covid-19 cases, hospital admissions, cases requiring intensive care, and deaths is in line with the increased coverage of Covid-19 booster and third doses in Sarawak,” Khairy said.
About 85 per cent of booster vaccine recipients in Sarawak took Pfizer, including those initially inoculated with Sinovac.
“This shows that use of the Pfizer vaccine as a heterologous booster dose is very effective. Besides that, no serious AEFI (adverse events following immunisation) have been reported, showing that heterologous vaccination is safe.”