KUALA LUMPUR, Dec 9 — The United States’ Food and Drug Administration (FDA) has endorsed the efficacy and safety of the Covid-19 vaccine by Pfizer and BioNTech, according to detailed documents released yesterday.
FDA reviewers have stated that the mRNA vaccine by US pharmaceutical company Pfizer and its German partner BioNTech, named BNT162b2, was “highly effective” in preventing symptomatic Covid-19, while data suggested the vaccine has a favourable safety profile, STAT reported.
The 53-page long briefing document from the FDA was released prior to tomorrow’s meeting of its independent Vaccines and Related Biological Products Advisory Committee, where FDA will get outside experts to give their opinion and vote if the benefits of the vaccine outweigh its risks. This is likely to be the final step before FDA grants emergency-use authorization (EUA) for this vaccine.
Usually, an EUA is normally not required to meet stringent requirements for approval. However, as the vaccine will be used by millions of people, the FDA is following its normal and tough advisory committee process, according to STAT.
The Pfizer-BioNTech vaccine’s data suggested that their two-dose vaccine can actually begin preventing Covid-19 even after its first dose. This could suggest that even if people miss their second dose, they still may have some form of protection from the coronavirus.
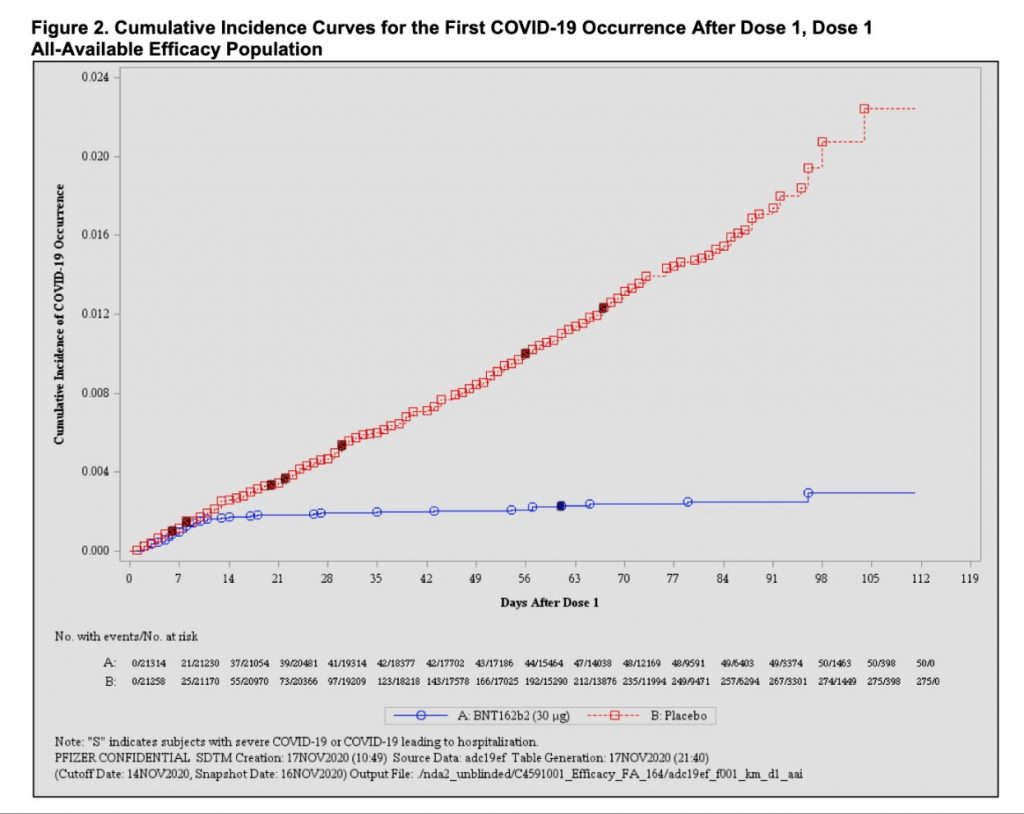
According to FDA’s document, which highlights the cumulative incidence of Covid-19 occurrence after dose one, the incidence line for those who received the first dose of the vaccine was almost plateau, while the incidence of those who developed Covid-19 after the placebo showed an increasing trend.
However, FDA scientists said that the results cannot support a conclusion on the efficacy of a single dose of the vaccine, as there wasn’t enough data to support it. The Pfizer-BioNTech Covid-19 vaccine is a two-dose regimen, with the second dose to be given three weeks after the first.
Vaccine 95% Effective At Cutting Covid-19 Infection Risk
Pfizer-BioNTech had included 44,000 people in their study, by which some were given placebo shots, while some were given the Covid-19 vaccine. The trial enrolled a diverse patient population in terms of race, ethnicity, age, and underlying health conditions.
From this total, there were 170 cases of symptomatic Covid-19, by which 162 of them received the placebo.
This means that eight people who received the Covid-19 vaccine had contracted the coronavirus. Hence, the vaccine has managed to reduce the risk of Covid-19 infection by 95 per cent.
There did not appear to be any difference in the vaccine’s efficacy based on gender, race, or those with conditions like obesity and hypertension.
STAT reported that Moderna’s study showed their vaccine can prevent severe Covid-19, as among the 30 cases who got severe Covid-19, none of them got their Covid-19 candidate vaccine, while in Pfizer’s study, 10 people developed severe Covid-19, out of which one of them received the vaccine.
The vaccine recipient who developed severe Covid-19 from Pfizer’s study had an oxygen saturation level of 93 per cent in room air, which meets the severe case definition but the number is barely below the normal level (97 per cent to 100 per cent). The subject was not hospitalised, did not seek further medical care, besides not having risk factors for severe disease.
In the briefing document, both Pfizer and FDA seemed to argue that perhaps this should not count against the vaccine.
Side Effects Of Pfizer-BioNTech Vaccine
The most common side effects from the vaccine highlighted in FDA’s briefing document were injection site reaction (84.1 per cent), fatigue (62.9 per cent), headache (55.1 per cent), chills (31.9 per cent), and fever (14.2 per cent).
As younger patients have stronger immune systems than older patients, 4.6 per cent under the age of 55 developed severe reactions, higher than 2.8 per cent of volunteers above the age of 55 with severe reactions.
Meanwhile, serious adverse events occurred in just half a per cent in the vaccine or placebo arms. There were four cases of Bell’s palsy (temporary weakness or paralysis of facial muscles), which occurred in those who got the vaccine.
A total of 64 cases were reported having lymphadenopathy (inflammation of the lymph nodes causing swelling), among which 58 were vaccine recipients, while six were from the placebo group.
The FDA has recommended Pfizer to follow vaccine recipients, specifically for the Bell’s palsy side effect. The FDA also urged Pfizer to keep patients blinded and not to offer placebo patients the vaccine, as what Pfizer intended to do once its vaccine receives EUA.
Pfizer and BioNTech have said that they will be able to manufacture 50 million doses of their vaccine for 25 million people by the end of this year. The US Centers for Disease Control and Prevention (CDC) is expecting 25 million doses of vaccine from Pfizer and 15 million doses from Moderna if they are authorised this month. This means that half of Pfizer’s vaccine produced this year will go to the US.
In 2021, Pfizer and BioNTech hope to manufacture up to 1.3 billion doses of its vaccine globally.








