KUALA LUMPUR, Dec 6 — Sinovac’s shot is the most expensive in the federal government’s Covid-19 vaccine portfolio, according to Permatang Pauh MP Nurul Izzah Anwar.
Nurul Izzah, who is a member of Parliament’s Public Accounts Committee (PAC), expressed concern over the high price of China’s inactivated vaccine, after government officials told the PAC in a meeting on August 2 that unpredictable delivery schedules led to changes in vaccine orders to meet demand.
For example, the PAC report revealed that the government made an additional order of two million Sinovac vaccine doses, after Pfizer failed to deliver the amount scheduled as planned by the Covid-19 Immunisation Task Force (CITF).
This resulted in the government paying more — a premium of between 16 and 18 per cent from the initial price agreed upon — for fresh orders of Sinovac’s finished product imported from China due to increased demand for vaccines and delays by Pharmaniaga Berhad in supplying its manufactured version.
“I was a bit worried because when we were given the details of the vaccine costs, of course the highest or among the highest was Sinovac. We cannot disclose here (in this meeting), officially.
“But in terms of effectiveness also with the available variants, although in general we say they are all effective, but it is different compared to vaccines coming from mRNA technology (such as Pfizer and Moderna),” Nurul Izzah said, based on transcripts of the meeting published in the PAC’s Covid-19 Vaccine Procurement report tabled in Parliament on December 1.
Malaysia’s local RECoVaM study by the Institute for Clinical Research showed that Sinovac’s inactivated vaccine was less effective than Pfizer’s mRNA shot in preventing infection, intensive care unit (ICU) admission, and death from Covid-19 within one to two months after two doses.
In three to five months after complete vaccination, Sinovac’s efficacy declined to 28 per cent against both infection and ICU admission, lower than the reduced effectiveness of Pfizer’s vaccine at 68 per cent and 79 per cent respectively in the same period.
The PAC was briefed about the prices of coronavirus vaccines procured by the government, but they can’t be disclosed publicly due to non-disclosure agreements.
Nurul Izzah asked health authorities at the August 2 PAC meeting if there were alternatives to the government’s reliance on Sinovac to fill orders, especially if Russia’s two-dose Sputnik V Covid-19 vaccine faces further delays in getting regulatory approval.
As of October 8, the government has procured 6.4 million doses of the Sputnik V vaccine, but has yet to administer them pending approval by the National Pharmaceutical Regulatory Agency (NPRA).
“So, I want to ask in terms of the approach by the Ministry of Health (MOH) to address this issue. Because it (the Sputnik V vaccines) is already here, there is already an agreement with Duopharma, but of course, if we cannot force… this is a realistic, a real problem.
“My question is, other than solely relying on Sinovac to fill in the gap… of course, we hope that Pfizer… they have been working very well, but by the looks of it, this (delayed NPRA approval for Sputnik V and supply shortage) is a real problem,” Nurul Izzah said.
Ministry of Health (MOH) secretary-general Mohd Shafiq Abdullah said in the event that the NPRA does not approve the Sputnik V Covid-19 vaccine by the Gamaleya Institute, the deal signed between Duopharma and Gamaleya will “automatically expire”.
NPRA director Dr Roshayati Mohamad Sani told the PAC that the dossier for Sputnik V has been submitted to the agency but many of its documentations were incomplete.
“On our part, we’ve had a discussion with the product registration holder (Duopharma), and twice with the product registration holder and Gamaleya. We also had a meeting with the Russian embassy to obtain information.
“They told us that certain information were confidential. How are we suppose to evaluate (the vaccine) if they are confidential?” Dr Roshayati said. She pointed out that the World Health Organization (WHO) has also been evaluating the Sputnik V vaccine since March.
The WHO also has not approved the Sputnik V vaccine. Russian Direct Investment Fund (RDIF) chief executive Kirill Dmitriev told Bloomberg TV last month that the Sputnik shot is on track to be approved by the WHO by the end of the year.
Most Covid-19 Vaccines Approved In A Month
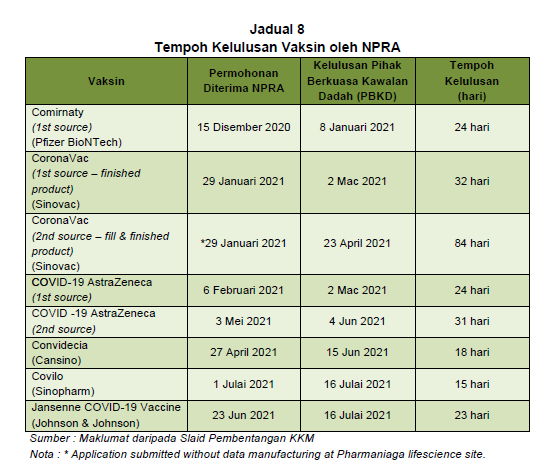
According to findings in the PAC report, NPRA approval for new drugs or vaccines typically takes 90 to 120 days under priority reviews granted during a pandemic, though it can be fewer than 90 days, depending on the pharmaceutical company’s response.
The NPRA took between 15 and 32 days to approve most Covid-19 vaccine brands from Pfizer to Johnson & Johnson. However, it took 84 days to approve Sinovac’s fill-and-finish product manufactured by Pharmaniaga.
In explaining NPRA’s approval process, Health director-general Dr Noor Hisham Abdullah told the PAC, in a meeting on January 5, that Malaysia cannot automatically follow decisions made by the United States’ Food and Drug Administration (FDA).
“What we’re doing now is to look at the quality and standards and we do not reciprocate from the FDA — meaning if the FDA approves, we will also approve. No, we don’t do that. We use the standards set by the NPRA. The problem arises when there is a death reported. If the NPRA doesn’t conduct the proper evaluation on the vaccine, do we then blame the FDA?
“We can’t blame the FDA. It will be our fault because we didn’t look at the data. Similarly at the Aseanlevel, we do not reciprocate but we do look at benchmarks to evaluate various vaccines and medicines.
“Once we have reached a certain benchmark, maybe we can reciprocate with Asean countries one day. But so far this is not the case. If it is approved in Singapore, it doesn’t necessarily mean it is approved in Malaysia? We still use NPRA standards. But the first step is to get a benchmark to evaluate the vaccine or the drugs.
“Because if there are complications, it is not the FDA or neighboring countries that will be responsible but we who are responsible for our people,” Dr Noor Hisham said.








