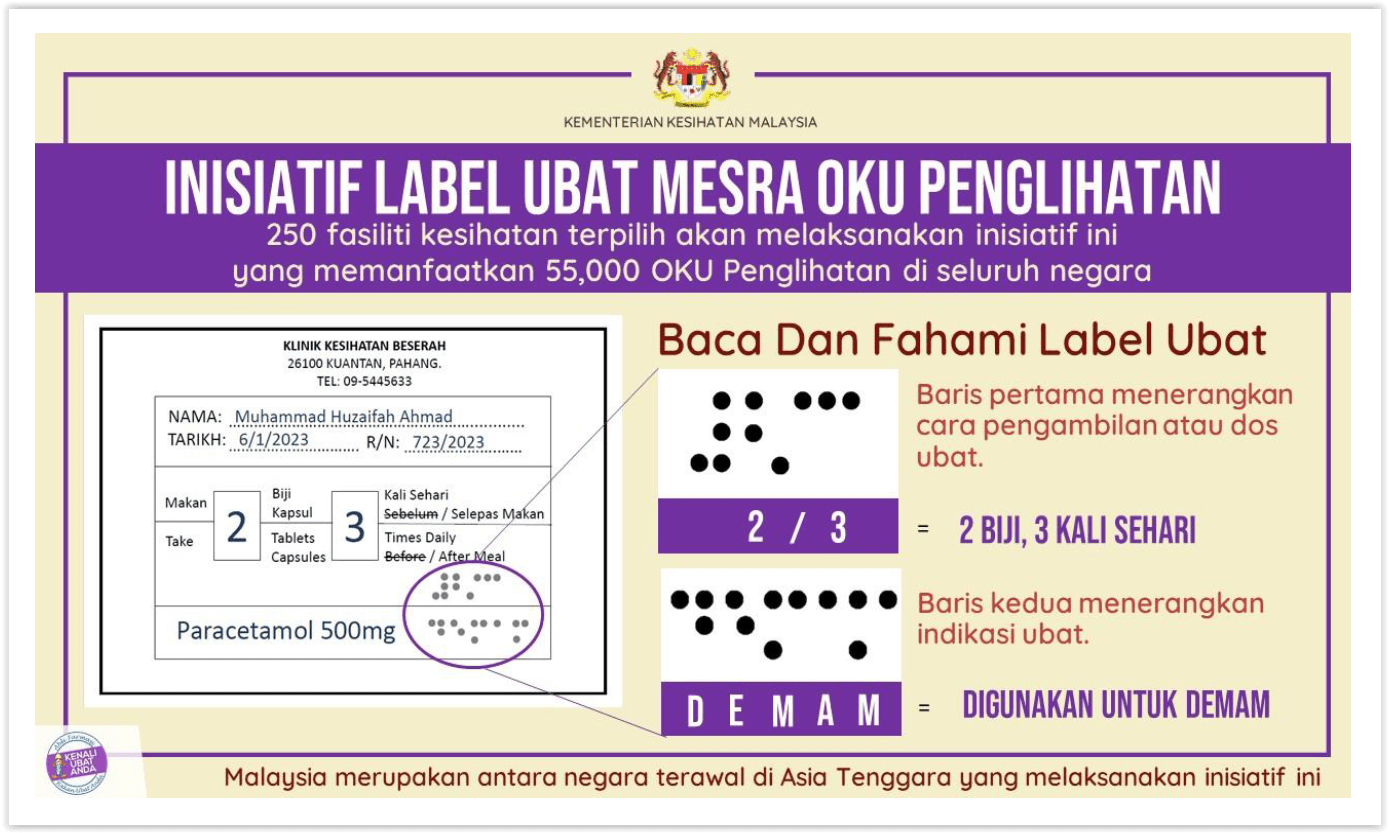
PUTRAJAYA, Jan 6 – The Health Ministry today launched an initiative to display pharmaceutical labels in Braille, but the name of the medicine is excluded from the tactile writing system for the blind.
Instead, the Braille format on pharmaceutical packaging in the Ministry of Health (MOH) initiative only includes dosage and indication, while the drug name remains in standard writing.
An example provided in Health Minister Dr Zaliha Mustafa’s statement of a label for paracetamol 500mg shows Braille for the dosage (two pills, three times a day) and indication (used for fever).
“Malaysia is among the earliest countries in Asia to introduce visually impaired OKU friendly medicine labels,” Dr Zaliha said in her 2023 new year address to MOH staff here today.
“This label was created to enable the visually impaired to get not just quick but accurate information about medicines and prescriptions for them. This is in line with the objective under Malaysian Patient Safety Goals 2.0 – Medication Safety: Medication without Harm.
“As a start, this initiative will be implemented in 250 public health clinics nationwide that will benefit 55,000 visually impaired people.”
Health director-general Dr Noor Hisham Abdullah confirmed with CodeBlue separately that the drug indication will be in Braille, but not the name of the medicine.
Under European law on the universal inclusion of Braille on pharmaceutical packaging in Europe, the name of the product must also be expressed in Braille format on the packaging.