KUALA LUMPUR, March 3 – Malaysia’s rate of reporting certain side effects from Covid-19 vaccination is very rare and much lower than in Australia, the United Kingdom, and Canada, according to the National Pharmaceutical Regulatory Agency (NPRA).
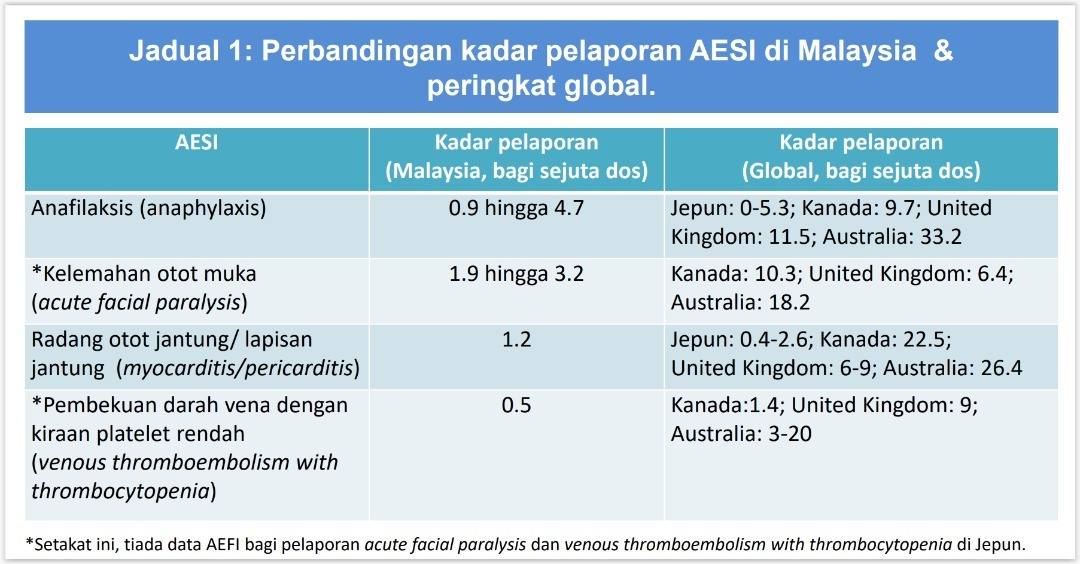
Based on a graphic shown by the NPRA at a media briefing yesterday, adverse events of special interest (AESI) following inoculation with the Covid-19 vaccine — anaphylaxis, acute facial paralysis, myocarditis or pericarditis, and venous thromboembolism with thrombocytopenia — were reported at lower rates per million vaccine doses in Malaysia compared to the three Western countries.
Australia reported the highest rates for all four AESI.
For example, anaphylaxis, which is a severe and potentially life-threatening allergic reaction, was reported at 0.9 to 4.7 reports per million doses in Malaysia, compared to 9.7 in Canada, 11.5 in the UK, and 33.2 in Australia. Malaysia’s reporting rate was similar to Japan’s rate of up to 5.3.
Acute facial paralysis was reported at 1.9 to 3.2 reports per million doses in Malaysia, compared to 6.4 in the UK, 10.3 in Canada, and 18.2 in Australia.
Myocarditis (inflammation of the heart muscle) or pericarditis (inflammation of the sac surrounding the heart) was reported at 1.2 reports per million doses in Malaysia, compared to 0.4 to 2.6 in Japan, six to nine in the UK, 22.5 in Canada, and 26.4 in Australia.
Malaysia reported 0.5 reports of venous thromboembolism with thrombocytopenia (blood clots with low platelet count) per million doses, compared to 1.4 in Canada, nine in the UK, and three to 20 in Australia.
NPRA acknowledged the possibility of Malaysia under-reporting adverse effects from the Covid-19 vaccine.
“The possibility is there because we’re doing passive reporting, not active reporting,” NPRA director Dr Roshayati Mohamad Sani told the press yesterday.
She urged Covid-19 vaccine recipients to report any side effects to a public or private health care facility when they seek treatment. The treating physician will then send a report to NPRA if their patients’ condition was related to vaccination.
NPRA pharmacovigilance division head Dr Azuana Ramli explained that passive surveillance of adverse events following immunisation (AEFI) means receiving reports of such cases, whereas active surveillance involves authorities looking for these cases.
“For example, in hospitals, we look for myocarditis cases and then you study it whether it’s linked to the vaccine or otherwise,” she said.
Regulatory bodies around the world, including NPRA, typically do passive surveillance of AEFI, she added.
Dr Azuana also highlighted behavioural differences in reporting adverse effects from vaccination and the ease of the reporting mechanism that could influence AEFI reporting rates.
“Of course, in any passive surveillance, the limitation is under-reporting. So we cannot deny there could be under-reporting,” she said.
Dr Azuana further cited international differences in reporting side effects from Covid-19 vaccination, explaining that people in other countries may report myocarditis based on their beliefs, even if their condition may not actually be myocarditis.
“So when they classify it, maybe out of 10 cases, only one is confirmed myocarditis. Whatever is reported in Australia, for example, could be gross myocarditis reported, but it’s actually not [myocarditis]. But in Malaysia, the ones we report, the cases are confirmed as myocarditis,” she said.
“It’s not really apple to apple when we compare.”
Dr Azuana said NPRA is currently conducting an active surveillance study with the Institute for Clinical Research to look for certain Covid-19 AEFI, such as myocarditis.