KUALA LUMPUR, Dec 13 — The National Pharmaceutical Regulatory Agency (NPRA) slashed its evaluation period for pharmaceutical products from a conventional 245 days to as short as six days for Covid-19 vaccines.
NPRA achieved this during the pandemic by placing more officers in charge of the coronavirus vaccine evaluation process and working “round-the-clock” to facilitate product registrations of the vaccines.
NPRA director Dr Roshayati Mohamad Sani told Parliament’s Public Accounts Committee (PAC) in a meeting on August 2 that mechanisms for fast-track conditional approvals for Covid-19 vaccines were put in place as early as December last year to reduce the evaluation period to between 30 and 90 working days.
“For regular products, it’s 245 days. There will be one or two officers in charge of evaluating the product, and they will work from 8am to 5pm, the usual working hours, five days a week. But with Covid-19 vaccines where we need them fast, we’ve placed nine officers (to evaluate the product) from all sections.
“From GMP to GSP, we’ve instructed all of them to assist as well. We’ve also asked our staff to work at irregular hours because I noticed that we sometimes receive emails from the product registration holders at 3am. So, as long as they can quickly approve the products.
“That’s why we push them hard because it’s a matter of great importance,” Dr Roshayati said, according to transcripts of the PAC meeting published in PAC’s Covid-19 Vaccine Procurement report tabled in Parliament on December 1.
Health director-general Dr Noor Hisham Abdullah, who was also present at the PAC meeting, said as more applications came in, the NPRA was able to speed up its authorisations further.
Dr Noor Hisham noted, however, that the vaccine approval duration also came down to how fast and complete the document submissions were by the submitting party. He said the evaluation period for vaccines under the COVAX facility that have been recognised by the World Health Organization (WHO) can be reduced further to fewer than 30 days.
The Ministry of Health (MOH), including the NPRA, was widely criticised for being “slow” in approving Covid-19 vaccines that led to subsequent delays in procurement.
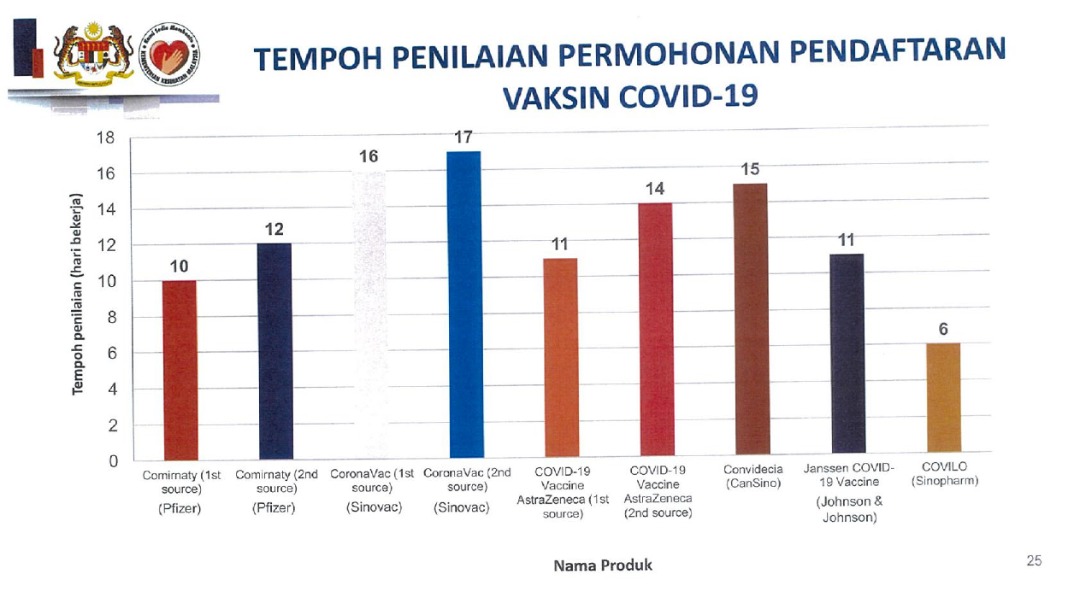
According to the MOH, NPRA’s evaluation period for the conditional approval of Covid-19 vaccines ranged from six to 17 days, with Pfizer’s approval taking 10 to 12 working days, AstraZeneca’s taking 11 to 14 days, and Sinovac taking 16 to 17 days. CanSino took 15 days, while authorisation for Johnson and Johnson and Sinopharm took 11 and six days respectively.
When compared against other countries like the United States and those in Europe where the Pfizer Covid-19 vaccine, for example, was approved in December 2020 versus Malaysia in January 2021, Dr Roshayati attributed the delay to the American pharmaceutical company not sending their dossier to NPRA soon enough.
“If we look at the timetable, we received Pfizer’s dossier on December 15 when, to compare, they sent their documents to the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) earlier in November.
“So, the later we receive the dossier, the later it will be for us at the NPRA to conduct our evaluation. They should have sent their dossiers earlier, and so should the others,” she said.
Dr Noor Hisham said that conditional approvals for Covid-19 vaccines will last for a year during which time, vaccine makers can continue to submit their latest data such as vaccine use and effectiveness for 12- to 15-year-olds.
Currently, the NPRA has yet to grant conditional approval for Gamaleya’s Sputnik V vaccine as requests for more data on the use of the Russian-made vaccine have yet to be met, Dr Noor Hisham said.
“There are two big issues, one is on the stability of that vaccine. That vaccine, probably in the colder, temperate countries, is probably good but in our tropic countries, it shows that [there is an] issue in terms of stability.
“If you take it out from the fridge, only two hours it would last. So that is where we are not sure of… two hours is – so, [it is a] crucial period of time [for us to know if the vaccine is] ineffective after two hours. So, we want more data on stability [but it was] not provided.
“Second is in terms of comorbidities. There is no mention about the vaccine to use [for people with] comorbidities, for example, diabetes, hypertension and what is the outcome of the vaccine. So, this is why we want more data on comorbid patients but [they have] not given it to us until today,” Dr Noor Hisham said.
The Sputnik V vaccine has also not received EMA approval, nor has the WHO listed the vaccine for use. “If the WHO has listed the vaccine, it takes about 30 days [before] we will approve the vaccine,” Dr Noor Hisham added.
Moving forward, he said the NPRA’s evaluation process will continue to improve to ensure that it is prepared to face the same scenarios come the next pandemic.
“So, the experience will provide us with the preparedness for the next pandemic. And our protocol, the way we do things now, never before we are looking into approval within 30 days or two weeks. But now the process continues to improve. And as I say, this is certainly an opportunity and we know that we can do it better,” he said.