This article is a follow-up to our earlier article on the vaccination of children 5 to 11 with the Pfizer vaccine.
Since we do not have risk benefit analysis for Covid-19 vaccination in children 5 to 11, it is best to refer to a study by the United States’ Food and Drug Administration (FDA).
This was unanimously passed by the FDA panel without opposition or abstentions. Similarly, it was evaluated by the Advisory Committee on Immunization Practices (ACIP), and unanimously recommended by all on the panel without exception.
They studied the risk benefit according to six different scenarios:
- Scenario 1 (Baseline Covid-19 Incidence)
- Scenario 2 (Covid-19 Peak Incidence)
- Scenario 3 (Lowest Covid-19 Incidence)
- Scenario 4 (Higher Vaccine Efficacy)
- Scenario 5 (Higher Covid-19 Death Rate)
- Scenario 6 (Lower Excess Myocarditis Rate)
Except for scenario 3, the model predicts that the benefits of the Pfizer Covid-19 vaccine given as a two-dose primary series clearly outweigh the risks for children ages 5 to 11 years.
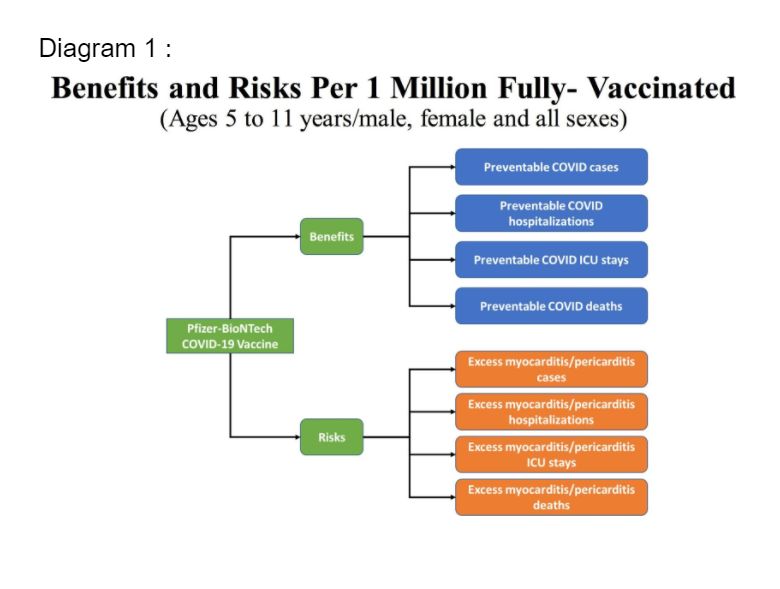
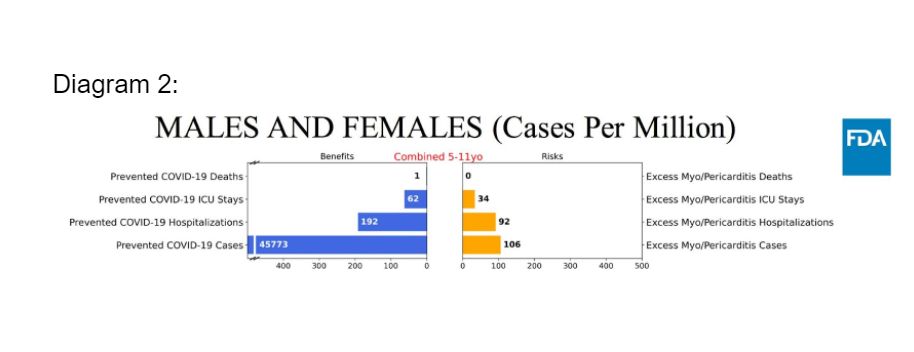
For scenario 3 (lowest Covid-19 incidence), the model predicts more excess hospitalisations and intensive care unit (ICU) admissions due to vaccine-related myocarditis and pericarditis, compared to prevented hospitalisations and ICU admissions due to Covid-19.
Under scenario 3, considering the different implications and lengths of hospital stays (six days) versus hospitalisation for vaccine-associated myocarditis or pericarditis (one day), and the benefits accrued from the prevention of Covid-19 cases with significant morbidities, the overall benefits of the vaccine still outweigh the risks.
The rates of myocarditis are based on data derived from adolescents and adults receiving a 30ug dose of the Pfizer vaccine. The dose for the pediatric group (children aged 5 to 11) is 1/3, i.e. 10ug dose. No cases of myocarditis occurred during the clinical trials for this group.
The underlying epidemiology of viral myocarditis is substantially lower in younger children compared to children aged 12 and older. It is anticipated that the rates of myocarditis and pericarditis after vaccination in children aged 5 to 11 are most likely lower than the rates quoted in the risk-benefit analysis.
The benefit of reducing Covid-related multisystem inflammatory syndrome in children (MIS-C) may not be fully captured by preventable hospitalisations, ICU admissions, and deaths due to Covid-19.
This risk assessment also does not consider potential long-term adverse effects due to long Covid, and does not include secondary benefits like reducing Covid-19 disease transmission.
It also does not include the benefits of preventing the emergence of more transmissible and virulent Variants of Concern (VOC).
The US Centers for Disease Control and Prevention (CDC) also compared the vaccination of children against other vaccine-preventable diseases (VPD) with the vaccination of children against Covid-19.
It is obvious that we are vaccinating against VPD, which have much lower mortality rates than that caused by Covid-19 in children.
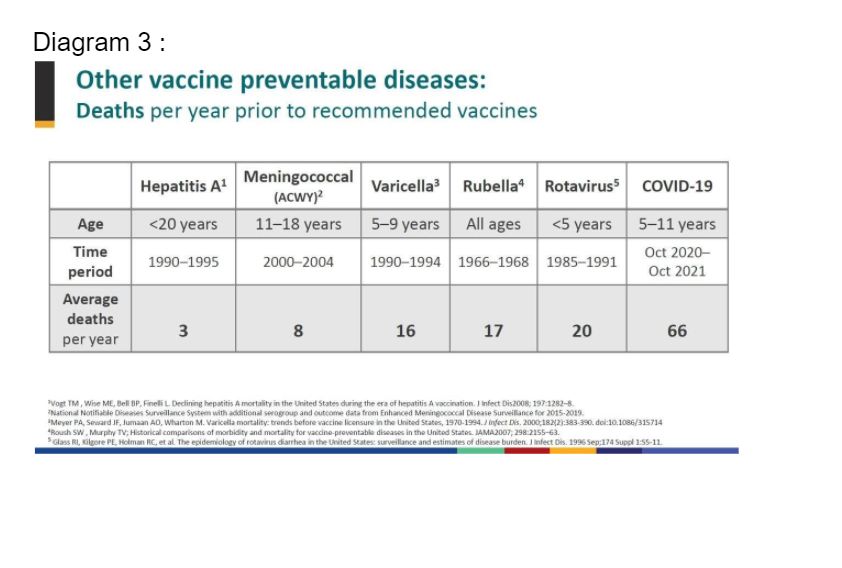
In summary, taking into account the six different scenarios, and considering the other benefits of reducing MIS-C, long Covid, disease transmission, emergence of virulent VOC, the utilisation of a much smaller dose of the vaccine, a safe return to school and social interactions, and the higher Covid-19 mortality rates versus other VPD, the benefits of the vaccine far outweigh any potential harmful effects.
- This is the personal opinion of the writer or publication and does not necessarily represent the views of CodeBlue.





