Many parents were dismayed by the recent announcement by health minister Khairy Jamaluddin that vaccines for children aged 5 to 11 years will only arrive at the end of June 2022.
This means that children who attend kindergarten and primary school will remain vulnerable for another six to eight months.
It is important to note that this delay is not due to a lack of effort, since Malaysia is one of the earliest to make a request to purchase the Pfizer vaccine for younger children.
But as we know, many developed nations are pushing to get the new Pfizer vaccine formulation for younger children, and hence, this pushes us lower in the queue, even though our negotiations occurred earlier.
We would like to discuss what options are available to us in this article. We will not discuss issues related to the safety of the use in young children. For such issues, we would like to refer documents and evidence by the United States’ Centers for Disease Control and Prevention (CDC) that outline efficacy, safety and risk-benefit.
In the past 16 weeks (four months), there have been 325,559 reported Covid-19 cases in children (87,829 in those aged 1 to 4, 132,436 in those aged 5 to 11, and 105,294 in those aged 12 to 17). Currently, 10.5 per cent of all new Covid-19 cases occur in children aged 5 to 11, according to CovidNow data.
This proportion has been increasing over time. As more adults and adolescents get vaccinated, and as schools and kindergartens open up, we can expect that unvaccinated children will have more Delta variant infections. Note that adolescents aged 12 to 17 now only account for 3.9 per cent of cases, due to the high rate of vaccine uptake.
At the time of writing, 116 children are reported to have died since the beginning of the pandemic (38 aged 1 to 4, 32 aged 5 to 11, and 46 aged 12 to 17). 85 per cent of deaths have occurred since July 2021, which displays the impact of the Delta variant on children.
Twenty-eight of the 32 deaths in the 5 to 11 age group occurred between July and October 2021 (seven per month), and this happened even with all schools and kindergartens closed. As primary schools and kindergartens reopen, we can expect seven to 10 deaths monthly, or possibly more, for the next six to eight months, for this age group.
This does not take into account the one in 100 infected with Covid-19 who will be hospitalised, with some seriously ill with multisystem inflammatory syndrome of children (MIS-C), and running the risk of acquiring long Covid.
Hence, if we do not vaccinate children aged 5 to 11 now, they are in for a difficult six to eight months ahead.
We must use every mitigation measure available to us to minimise the risk of Covid-19 infection in our children. This means making sure that kindergartens and primary schools have excellent ventilation, ensuring that every adult around them is vaccinated, maintaining good filtration and fit mask use, using classroom bubbles, limiting unnecessary social contact, promoting hand disinfection, and continuing to maintain physical distancing.
One suggestion has been to use the Pfizer vaccine for teenagers and adults in children aged 5 to 11 at a smaller dose. Is this feasible? The vaccine approved for children aged 5 to 11 is identical to that approved for older persons, except that we administer 10mcg, instead of 30mcg (one third the adult dose).
This would mean administering a 0.1 ml dosage of the adult and adolescent formulation, but the logistics involving these small volumes are not unprecedented. For decades, our nurses have been administering 0.1 ml of IM Vitamin K and intradermal 0.05 ml of the BCG vaccine. Training of nurses and vaccinators will be required for them to be familiar with the small volumes.
However, this reduced dose of the Pfizer vaccine for young children will need to be repackaged, requiring not just a lower dose schedule, but also ensuring that it contains a different buffer, allowing for longer refrigeration at higher temperatures (see graphic below). This can be stored at a refrigerator temperature of 2-8 Celsius for up to 10 weeks.
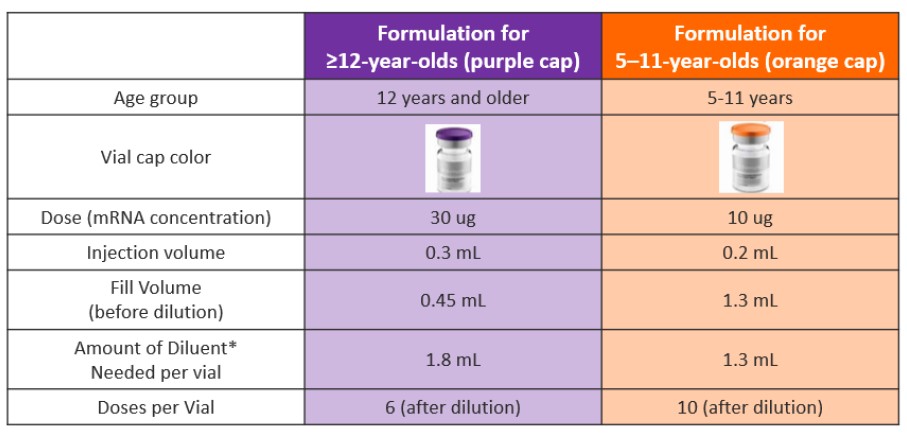
Hence, it is the same vaccine, just repackaged. If we use the adult formulation, it will be considered as off-label use by the company, which means using at your own risk, opening up governments to possible litigation.
Many parents we have spoken to are keen to get the vaccine now, rather than wait six to eight months for their children. The preventable death of a single child is unacceptable to any parent or health care professional.
If we opt to take this approach, we will need parental understanding and consent. As with the delivery of any vaccine or drug, care needs to be taken to avoid any errors in administering doses, i.e. making sure children get only a 10mcg dose.
The National Pharmaceutical Regulatory Agency (NPRA) will need to give conditional approval for such a move. In addition, it must also keep a close watch on vaccine safety signals (side effects), and act early if any should appear. Parents should be notified of any possible side effects.
A minimal approach is to vaccinate all children aged 5 to 11. Of the 116 children who have died, 83 per cent had comorbidities. So we could offer the adult formulation (using a 10mcg dose) for all children with chronic illnesses and disabilities in order to reduce deaths.
However, it is important that any approach we take should not deprive older people from booster doses.
Different nations are using different vaccines to extend protection to young children. The United States has started vaccinating young children in November 2021 with Pfizer. Israel has approved Pfizer and hopes to start very soon.
Singapore hopes to get the new Pfizer formulation by January 2022, and extend vaccination to young children. European Union medical regulators have started evaluating the use of Pfizer in young children.
China has authorised use of Sinovac and Sinopharm vaccines for children aged 3 to 17. The United Arab Emirates has approved Sinopharm for children aged 3 to 17 and Pfizer for children aged 5 to 11.
Argentina is vaccinating children as young as 3 years old with the Sinopharm vaccine. Indonesia, Chile and Ecuador have approved the Sinovac vaccine for children aged 6 and above.
Although Phase 1 and 2 clinical trials on the use of Sinovac for young children have been published, we have yet to see data on Phase 3 trials (unlike Pfizer).
Sinovac has been given to more than 60 million teenagers in China, however, no safety data has been made public. It would be good to get Phase 3 efficacy and safety data on Sinovac before it is authorised for use in children.
The Ministry of Health (MOH) has embarked on a Phase 3 clinical trial using the Sinovac vaccine for children aged 3 to 11, but the results of this will only be available many months from now.
Our children have been living through not only the Covid-19 pandemic but also a mental health pandemic. The enormous disruption in schooling in Malaysia (which counts among the longest in the world) and loss of social interaction has taken a serious toll on children.
Many are anxious and lonely. Others have become disinterested in school and have regressed in their learning. Some have trouble with sleep.
Getting our children safely back to school is one way of dealing with some of these concerns. Vaccination is one strategy to make this happen.
Data has shown that the benefits of vaccination outweigh the risks. Vaccinating children will also benefit the larger society and reduce the spread of Covid-19, minimising breakthrough infections and serious illness in vulnerable adults.
We hope this article will open a dialogue that can enable the early vaccination of our children. Parents should advocate for the options suggested here.
If there is no way for the authorities to expedite the Pfizer vaccine formulation for children, then we ask the MOH and the NPRA to consider this option of using 0.1ml of the adult formulation, with appropriate training and briefing of the health care workers involved.
- This is the personal opinion of the writer or publication and does not necessarily represent the views of CodeBlue.




