This narrative may not go down very well with the many young Malaysians who have successfully secured their AstraZeneca (AZ) doses.
It was a frantic scrambling by the tech-savvy for the 268,000 doses of the AZ vaccine. All vaccine doses were snapped within four hours.
Now that they have jumped the queue, some of them have even begun to rationalise the legitimacy of the first-come first-served vaccine rollout.
Due to the limited supplies of vaccines, like all other global health authorities and agencies, the Ministry of Health (MOH) and the Special Committee for Ensuring Access to Covid-19 Vaccine Supply (JKJAV) phased the vaccine rollout based on three major considerations:
- To protect and preserve the functioning of our society, namely the health care and essential services.
- To decrease serious Covid-19 disease and deaths as much as possible.
- To reduce the added burden of Covid-19 disease on people facing disparities.
These considerations are premised on four major ethical principles:
- The allocation of the scarce vaccine supplies should be aimed at maximising the benefits and minimising the harms. The reduction of Covid-19 associated morbidity and mortality would reduce the burden on the health care capacity. The most vulnerable in terms of inevitable work exposure and the other high-risk groups for Covid-19 should be determined by scientific evidence.
- To promote justice to advance equal opportunities for everyone to enjoy optimum health and wellbeing as vaccines become more widely available.
- To mitigate health inequities and allow everyone the opportunity to attain full health potential and not be disadvantaged due to any social determinants.
- To promote transparency in the decision-making process to inspire public trust in the implementation of the vaccine rollout.
Pivoting on these incontrovertible principles, during the period of limited vaccine supply, the MOH and JKJAV have identified five major groups:
- Health care workers.
- Critical and essential services personnel.
- Senior citizens.
- Persons with comorbidities.
- The physically and intellectually challenged (OKU).
One does not sacrifice pristine ethical values simply based on a perception that there is “public fear over the AZ vaccine” because “we understand that is what the public feels”.
Where may I ask is the scientific evidence to substantiate this personal perception? From the outset, there has always been a substantial proportion of the citizens who have been either vaccine-hesitant or resistant. This has remained fairly consistent for the period of December 2020 to February 2021.
The US Food and Drug Administration (FDA) paused the Johnson & Johnson (J&J) vaccine (adenoviral vector mode of action like the AZ vaccine) for 10 days to investigate a possible link with blood clots. Unlike JKJAV, they undertook a 50 state survey which showed that:
- There was high awareness of the J&J vaccine pause.
- Vaccine hesitancy and refusal did not increase.
- The pause did not have any major negative effects on vaccine preferences and attitudes.
During the period of December 2020 to April 2021, when the AZ and J&J vaccines were being investigated, except for the UK, overall, there was no decrease in vaccine acceptance in eight European countries.
It would seem that the scientific studies and surveys do not validate the presumptions of the MOH or JKJAV. But if they insist that the socio-demography of vaccine-hesitant and resistant Malaysians are diametrically different from their counterparts in the US and eight other European countries, then they ought to provide us with the evidence.
The Centers for Disease Control and Prevention (CDC) and FDA paused the use of the J&J vaccine following reports of six cases (one fatal) of cerebral venous sinus thrombosis (CVST) with thrombocytopenia, on April 13, 2021, and promptly issued a health alert.
The Advisory Committee on Immunization Practices (ACIP) met the following day, illustrating a sense of urgency in investigating the association of the J&J vaccine with the blood clots, a.k.a. Thrombocytosis Thrombocytopenia Syndrome (TTS).
Within ten days, the ACIP presented its findings and recommended to the CDC to lift the pause on the J&J vaccine for use in adults.
We are made to understand that there was an assessment committee to review the AZ vaccine, its plausible association with TTS and its utilisation in the Malaysian context. We would appreciate if the MOH or JKJAV can point us to the conclusions of the study and its recommendations.
This is in part fulfilment of the ethical principles of transparency and accountability which would inspire confidence among the medical associations and professionals that a thorough analysis of the evidence has been undertaken and contextualised for the National Covid-19 Immunisation Programme (PICK).
This would then facilitate an appropriate and comprehensive risk communications about the AZ vaccine. Some feel that this effort was not forthcoming from the nation’s highest health and vaccine authorities.
An individual who actually took the initiative to frame “Key FAQs: A set of crucial questions you may want answered before deciding to opt in or out of the AZ vaccine”, tweeted, “The health ministry couldn’t have been sloppier by just dumping a page of links at us.”
So what is the scientific and ethical basis for the MOH and JKJAV to offer the AZ vaccine “on a first come, first served basis to the public aged above 18 who are willing to have it”?
Britain has the largest experience with the AZ vaccine. More than 50 million doses have been utilised, a substantial proportion of which were AZ doses.
Following the first dose of the AZ vaccine for the elderly, 90 per cent of Covid-19 associated hospitalisations was reduced. The UK Joint Committee on Vaccination and Immunization had prioritised the 65-and-above age group for the AZ vaccine doses.
And for the four-month period from December 2020 to March 2021, Public Health England estimated that 10,400 deaths have been prevented in persons above 60 years old in the UK.
The high vaccine efficacy claimed in the clinical trains have now been vindicated through real-world experience.
With access to probably the largest data-set for the AZ vaccine rollout, the UK Joint Committee on Vaccination and Immunization recommended, “based on available data and evidence, it was preferable for adults aged under 30 with no underlying conditions to be offered an alternative to the AZ vaccine where available”.
How does the MOH and JKJAV scientifically and ethically justify providing the AZ vaccine to young adults aged 18 tp 30, considering that the UK, with the largest and widest experience, with its homegrown University of Oxford vaccine, has advised otherwise?
An interim risk benefit analysis of the AZ vaccine and TTS can be accessed here. It references the European Medicines Agency (EMA) which has meticulously analysed the data to stratify the very rare risk of blood clots with the benefits for different age groups and Covid-19 incidence rates.
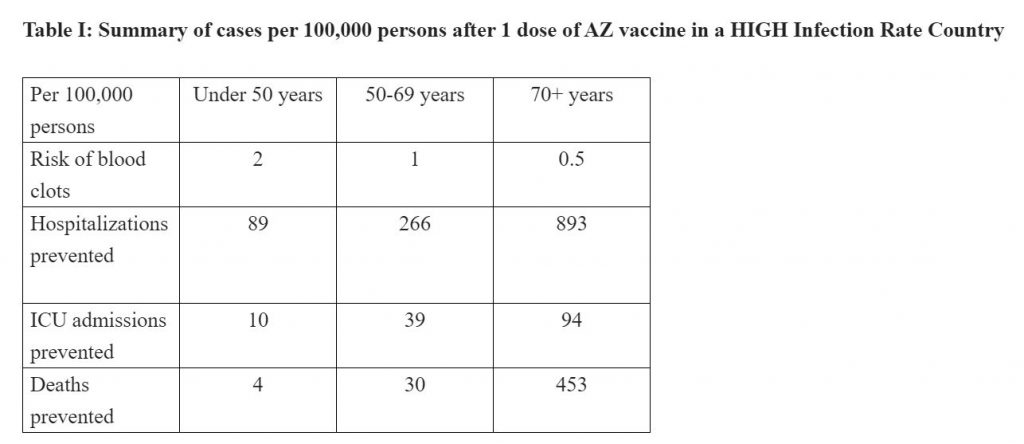
The four to five per million often touted is actually the risk of blood clots in persons aged above 70, as per the EMA analysis. The risk is actually four times higher in persons below 50, i.e. 20 per million.
The risk of being admitted to hospital and of dying from Covid-19 in a 70-year-old is 1,786 and 906 times respectively, when compared to the risk of suffering from a blood clot.
Thus my suggestion to prioritise the AZ vaccine rollout in the high infection rate states, namely Sarawak, Kelantan, Kuala Lumpur and Selangor, with the first right of refusal for the groups with the highest risk of severe Covid-19 disease and deaths, i.e. those above 60 years old.
Only when these high-risk elders have been protected, then the AZ vaccine can be offered to others. This makes medical sense and it is the ethically right response, within the context of limited vaccine supplies and the presently known risk benefit analysis.
It is probably the less-than-impressive odds in the younger age groups that have persuaded many health authorities in European countries to reserve the AZ vaccines for those above 50.
Unless the MOH and JKJAV has evidence to the contrary, the deluge of young would-be vaccinees may precipitate a more-than-usual load of life-threatening TTS cases in our already overwhelmed health care facilities.
- This is the personal opinion of the writer or publication and does not necessarily represent the views of CodeBlue.







